Literature review
Published in spanish Científica Dental Vol. 21. Nº 2. 2024
www.cientificadental.es
Osteonecrosis of the jaws in patients treated with monoclonal antibodies: A review of the literature
Introduction: In the last decade, monoclonal antibodies have revolutionized the field of modern medicine. These are proteins designed to bind specifically to certain molecules for the treatment of certain types of cancer and autoimmune disease.
The aim of this study was to analyze the relationship between treatment with monoclonal antibodies and osteonecrosis of the jaws by analyzing the incidence and associated risk factors.
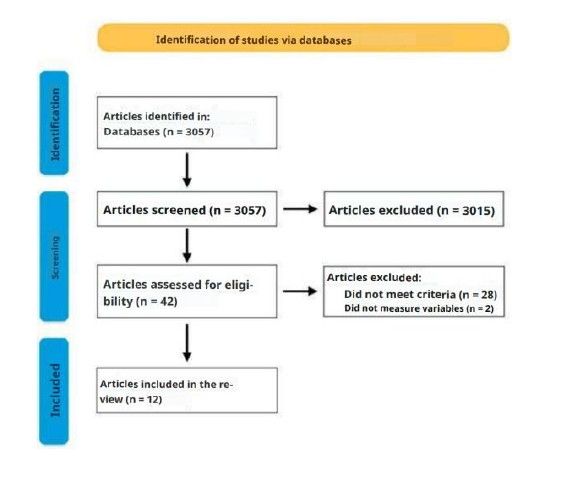
Methods: A total of 3057 results were initially obtained. After an initial screening of articles that did not meet the inclusion criteria, 42 articles were selected for full-text reading. Finally, 13 randomized clinical trials were included.
Results: The total number of patients included was 16259. The mean incidence of osteonecrosis cases was 3.87%. There were 51 mild cases of osteonecrosis (stage 1-2) and 16 severe cases (stage 3). The risk factors analyzed were the use of mismatched prostheses, invasive dental procedures, periodontal disease and the use of corticosteroids.
Conclusions: The mean incidence of monoclonal antibody-induced osteonecrosis was 3.87%. The use of misaligned prostheses, tooth extraction, periodontal disease and the use of corticosteroids may favor the development of monoclonal antibody-induced osteonecrosis.
The mean incidence of monoclonal antibody-induced osteonecrosis was 3.87%. The use of misaligned prostheses, tooth extraction, periodontal disease and the use of corticosteroids may favor the development of monoclonal antibody-induced osteonecrosis.
Studies of higher quality and with longer follow-up time are necessary to reach more conclusive statements.
Monoclonal antibodies (MAs) are molecules that act as substitutes for endogenous antibodies to restore, enhance, or mimic the activity of the immune system1.
MAs have revolutionised the treatment of autoimmune, allergic, and infectious diseases, being useful in cases of multiple sclerosis, bone metastases, and osteoporosis2-4.
Four types of monoclonal antibodies are distinguished according to their origin: murine, chimeric, humanised, and human. The most commonly used and prescribed at present are the humanised antibodies, identified by the suffix -zumab (romosozumab), and the human antibodies, identified by the suffix -umab (denosumab); the latter are less antigenic, better tolerated, and possess a longer halflife. Both act by inhibiting osteoclast activity, thereby reducing bone resorption and increasing bone density3, with a highly specific mechanism of action through inhibition of the receptor activator of nuclear factor-kappa B ligand (RANKL)5.
RANKL is a critical factor in bone resorption, as it plays a fundamental role in the formation, function, and survival of osteoclasts. The RANKL inhibitor is osteoprotegerin, which, similarly to monoclonal antibodies, competes with RANKL for binding to RANK, thereby neutralising its
effects. Thus, inhibition of RANKL permits an increase in bone density6,7.
Owing to their mechanism of action, these agents may have several adverse effects, including increased susceptibility to infections, hepatic injury, and osteonecrosis of the jaws (ONJ), which is an uncommon but serious condition characterised by one or more necrotic bone lesions that are exposed or can be palpated through an intraoral or extraoral fistula in the maxillofacial region, and persist for at least 8 weeks without response to appropriate treatment8,9.
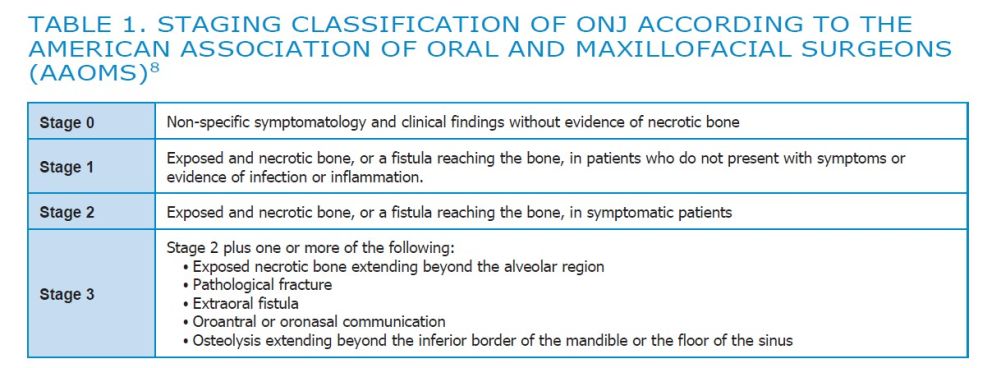
The American Association of Oral and Maxillofacial Surgeons (AAOMS) introduced the staging system to classify the symptomatology of ONJ and facilitate decision-making for its treatment8. (Table 1)
articles
In the past decade, the use of monoclonal antibodies has increased; therefore, the aim of this literature review was to analyse the association between monoclonal antibody therapy and the incidence of ONJ, as well as the risk factors in patients with ONJ treated with monoclonal antibodies.
Sources and search strategy: A literature search was
conducted using the PubMed/Medline database,
employing the following keywords: [(monoclonal
antibodies) OR (antiresorptive drugs)] AND
[(osteonecrosis of the jaw) OR (ONJ)].
Inclusion criteria: Randomised controlled trials (RCTs) from the past 10 years describing the incidence of ONJ in patients treated with monoclonal antibodies (MAs) were included.
Exclusion criteria: In vitro studies, animal studies, and observational studies were excluded. RCTs reporting ONJ caused by drugs other than MAs were also excluded.
Selected articles: Following the initial search, a total of 3,057 results were obtained. An initial screening was conducted, excluding articles that did not meet the inclusion criteria based on title and abstract.
Subsequently, 42 articles were read in full, and ultimately, 12 articles were included in the review (Figure).
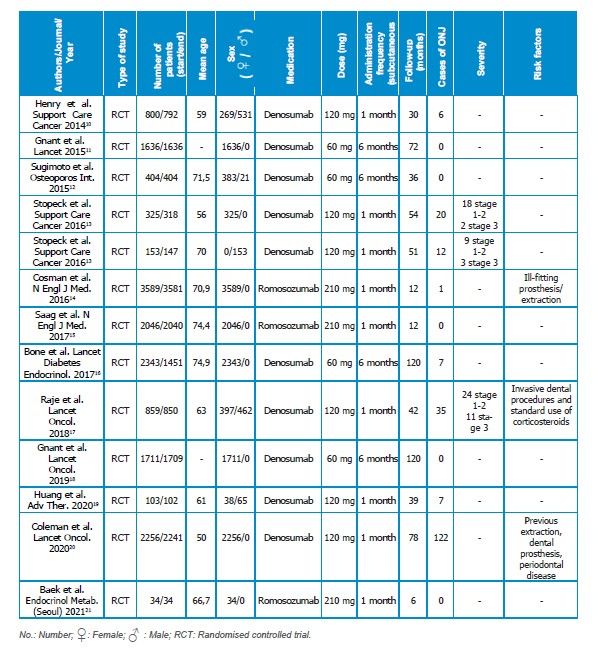
Information recorded from the articles: The names of the authors, year of publication, number, sex and age of the patients, follow-up period, type, dose and frequency of administration of the monoclonal antibody (MA) used, number of reported cases, severity, and risk factors were recorded.
In total, 13 RCTs were analysed, as the article by Stopeck et al.13 presented two studies. The total number of patients was 16,259, 15,027 women and 1,232 men, with a mean age of 65.22 years.
The most frequently used MA was denosumab, which was analysed in 10 studies and administered at doses ranging from 60 mg every 6 months to 120 mg monthly. The other MA, analysed in three studies, was romosozumab, administered at a dose of 210 mg monthly. The follow-up period for patients ranged from 6 to 120 months.
In five studies11,12,15,18,21 no cases of ONJ were
reported, whereas in the remaining seven
studies10,13,14,16,17,19,20 an incidence ranging from 0.028% to 8% (mean 3.87%) was observed. The severity of ONJ cases was analysed in only three studies13,17, with 51 mild cases (stage 1–2) and 16 severe cases (stage 3) reported. Finally, the factors associated with the development of ONJ were analysed in three studies14,17,20 in which the use of ill-fitting prostheses, extractions, invasive dental procedures, periodontal disease, and the use of
corticosteroids were described (Table 2).

AMERICAN ASSOCIATION OF ORAL AND MAXILLOFACIAL SURGEONS
(AAOMS)8
ONJ is a rare pathology that considerably impairs
patients’ quality of life. In this literature review, 12 studies were included in which cases of patients
receiving monoclonal antibody therapy were
documented, with denosumab (60 mg every 6
months)11,12,16,18 being the most frequently utilised.
The majority of cases studied were women (92.4%),
which may be explained by the high prevalence of
osteoporosis following menopause. The most commonly utilised treatments for osteoporosis are zoledronic acid, denosumab, and teriparatide, as they demonstrate high efficacy in reducing the risk of bone fractures22.
ONJ is most frequently localised in the mandible22,23; however, it may also be detected in the maxilla24.
Furthermore, it may be accompanied by pain,
inflammation, erythema, suppuration, and tooth loss.
Although ONJ may occur spontaneously, in the majority of cases it results from a surgical procedure in the oral cavity25.
With regard to the incidence of ONJ, a variation between 0% and 8% was observed, which may be attributable to differences in sample sizes among the studies and the follow-up period. Furthermore, when comparing the mean incidence obtained in this review (3.87%) with other drugs that may also induce ONJ, such as intravenous bisphosphonates (1.3–3.2% after 3 years of follow-up) and oral bisphosphonates (between 1–2.3% after 3 years of follow-up, the incidence of MRONJ is observed to be higher in patients taking antiresorptive agents (ARs) 26,27. This increased incidence was already noted by Loyson et al.28, who confirmed a higher risk of MRONJ in patients who switched from bisphosphonates to ARs. It is worth noting, however, that the effects of bisphosphonates on bone can last up to three years after the last dose, unlike ARs, which do not have a cumulative effect22. The risk factors for ONJ associated with monoclonal antibody therapy were described in three studies14,17,20. The risk factors for ONJ related to the use of monoclonal antibodies that were identified are similar to those for ONJ induced by bisphosphonates: use of ill-fitting prostheses, extractions, invasive dental procedures, periodontal disease, and use of corticosteroids. Additionally, ONJ caused by bisphosphonates presents further risk factors such as the cumulative dose of bisphosphonates in the blood and tobacco use29.
In this context, it is important to implement a review programme for patients treated with monoclonal antibodies, as the majority of diagnosed cases of ONJ associated with monoclonal antibody therapy are mild (stages 1–2). Seventy-six per cent of cases in which the stage is recorded are mild, thereby underscoring the particular importance of early diagnosis of ONJ30.
With regard to the management of ONJ, the literature describes adjuvant treatments (antibiotics, oral rinses) for mild cases (stages 1–2). In stage 3, for those cases that do not respond to adjuvant treatment, surgical procedures (debridement, curettage, removal of sequestra, and bone resection) should be employed, ensuring complete removal of necrotic bone, smoothing of the bone margins, and meticulous wound closure31.
Other therapeutic alternatives are currently under investigation, such as the use of platelet concentrates, teriparatide, laser therapy, hyperbaric oxygen, and ozone applications. These therapies may be effective, although at present they exhibit a low level of evidence and a limited sample size32.
One of the limitations of the present review is the short follow-up period (< 5 years) in nine of the thirteen included studies. Furthermore, in eight of the thirteen included studies, the sample comprises exclusively women; thus, it would be of interest to determine the incidence according to gender. Finally, it would be beneficial to compare the incidence of ONJ between monoclonal antibodies and other antiresorptive agents.
The incidence of ONJ induced by monoclonal antibodies in this review is higher than that of other agents, such as oral and intravenous bisphosphonates. Furthermore, it appears that the use of ill-fitting prostheses, extractions,
periodontal disease, and corticosteroid use may promote the development of ONJ associated with the administration of monoclonal antibodies. Nevertheless, further randomised clinical trials comparing monoclonal antibodies with other antiresorptive agents are required to more precisely determine the incidence, severity, and risk
factors.
Bayer V. An overview of monoclonal antibodies. Semin Oncol Nurs. 2019;35(5):150927.
Grilo AL, Mantalaris A. The increasingly human and profitable monoclonal antibody market. Trends Biotechnol. 2019;37(1):9-16.
Delmas PD. Clinical potential of RANKL inhibition for the management of postmenopausal osteoporosis and other metabolic bone diseases. J Clin Densitom. 2008;11(2):325-338.
Cummings SR, San Martin J, McClung MR, et al. Denosumab for prevention of fractures in postmenopausal women with osteoporosis. N Engl J Med. 2009;361(8):756-765.
Boquete-Castro A, Gómez-Moreno G, Calvo-Guirado JL, et al. Denosumab
and osteonecrosis of the jaw. A systematic analysis of events reported in clinical trials. Clin Oral Implants Res. 2016;27(3):367-375.
Capparelli C, Morony S, Warmington K, et al. Sustained antiresorptive effects
after a single treatment with human recombinant osteoprotegerin (OPG): a pharmacodynamic and pharmacokinetic analysis in rats. J Bone Miner Res. 2003;18(5):852-858.
Kostenuik PJ. Revisiting the seed and soil theory of bone metastasis: new tools, same answer. J Musculoskelet Neuronal Interact. 2004;4(4):375-376.
Ruggiero SL, Dodson TB, Aghaloo T, et al. American Association of Oral and Maxillofacial Surgeons’ position paper on medication-related osteonecrosis of the jaws-2022 Update. J Oral Maxillofac Surg. 2022;80(5):920-943.
McGowan K, McGowan T, Ivanovski S. Risk factors for medication-related osteonecrosis of the jaws: A systematic review. Oral Dis. 2018;24(4):527-536.
Henry D, Vadhan-Raj S, Hirsh V, et al. Delaying skeletal-related events in a randomized phase 3 study of denosumab versus zoledronic acid in patients with advanced cancer: an analysis of data from patients with solid tumors. Support Care Cancer. 2014;22(3):679-687.
Gnant M, Pfeiler G, Dubsky PC, et al. Adjuvant denosumab in breast cancer (ABCSG-18): a multicentre, randomised, double-blind, placebo-controlled trial. Lancet. 2015;386(9992):433-443.
Sugimoto T, Matsumoto T, Hosoi T, et al. Three-year denosumab treatment in postmenopausal Japanese women and men with osteoporosis: results from a 1-year open-label extension of the Denosumab Fracture Intervention Randomized Placebo Controlled Trial (DIRECT). Osteoporos Int. 2015;26(2):765-774.
Stopeck AT, Fizazi K, Body JJ, et al. Safety of long-term denosumab therapy: results from the open label extension phase of two phase 3 studies in patients with metastatic breast and prostate cancer. Support Care Cancer. 2016;24(1):447-455.
Cosman F, Crittenden DB, Adachi JD, et al. Romosozumab treatment in postmenopausal women with osteoporosis. N Engl J Med. 2016;375(16):1532-1543.
Saag KG, Petersen J, Brandi ML, et al. Romosozumab or alendronate for fracture prevention in women with osteoporosis. N Engl J Med. 2017;377(15):1417-1427.
Bone HG, Wagman RB, Brandi ML, et al. 10 years of denosumab treatment in postmenopausal women with osteoporosis: results from the phase 3 randomised FREEDOM trial and open-label extension. Lancet Diabetes Endocrinol. 2017;5(7):513-523.
Raje N, Terpos E, Willenbacher W, et al. Denosumab versus zoledronic acid in bone disease treatment of newly diagnosed multiple myeloma: an international, double-blind, doubledummy, randomised, controlled, phase 3 study. Lancet Oncol. 2018;19(3):370- 381.
Gnant M, Pfeiler G, Steger GG, et al. Austrian Breast and Colorectal Cancer Study Group. Adjuvant denosumab in postmenopausal patients with hormone receptor-positive breast cancer (ABCSG-18): disease-free survival results from a randomised, double-blind, placebo-controlled, phase 3 trial. Lancet Oncol. 2019;20(3):339-351.
Huang SY, Yoon SS, Shimizu K, et al. Denosumab versus zoledronic acid in bone disease treatment of newly diagnosed multiple myeloma: An international, double-blind, randomized controlled phase 3 study-asian subgroup analysis. Adv Ther. 2020;37(7):3404- 3416.
Coleman R, Finkelstein DM, Barrios C, et al. Adjuvant denosumab in early breast cancer (D-CARE): an international, multicentre, randomised, controlled, phase 3 trial. Lancet Oncol. 2020;21(1):60-72.
Baek KH, Chung YS, Koh JM, et al. Romosozumab in postmenopausal korean women with osteoporosis: A randomized, double-blind, placebocontrolled
efficacy and safety study. Endocrinol Metab (Seoul). 2021;36(1):60-69.
Nicolatou-Galitis O, Schiødt M, Mendes RA, et al. Medication-related osteonecrosis of the jaw: definition and best practice for prevention, diagnosis, and treatment. Oral Surg Oral Med Oral Pathol Oral Radiol. 2019;127(2):117-135.
Pazianas M, Miller P, Blumentals WA, et al. A review of the literature on
osteonecrosis of the jaw in patients with osteoporosis treated with oral bisphosphonates: prevalence, risk factors, and clinical characteristics. Clin Ther. 2007;29(8):1548-1558.
Filleul O, Crompot E, Saussez S. Bisphosphonate-induced osteonecrosis of the jaw: a review of 2,400 patient cases. J Cancer Res Clin Oncol. 2010;136(8):1117-1124.
Eguia A, Bagán-Debón L, Cardona F. Review and update on drugs related
to the development of osteonecrosis of the jaw. Med Oral Patol Oral Cir Bucal. 2020;25(1):e71-e83.
Limones A, Sáez-Alcaide LM, Díaz-Parreño SA, et al. Medication-related
osteonecrosis of the jaws (MRONJ) in cancer patients treated with denosumab VS. zoledronic acid: A systematic review and meta-analysis. Med Oral Patol Oral Cir Bucal. 2020;25(3):e326-e336.
Kawahara M, Kuroshima S, Sawase T. Clinical considerations for medicationrelated
osteonecrosis of the jaw: a comprehensive literature review. Int J Implant Dent. 2021;7(1):47.
Loyson T, Van Cann T, Schöffski P, et al. Incidence of osteonecrosis of the jaw in
patients with bone metastases treated sequentially with bisphosphonates and denosumab. Acta Clin Belg. 2018;73(2):100-109.
Nisi M, La Ferla F, Karapetsa D, et al. Risk factors influencing BRONJ staging in patients receiving intravenous bisphosphonates: a multivariate analysis. Int J Oral Maxillofac Surg. 2015;44(5):586-591.
Al Abdullateef A, Alhareky MS. Awareness among patient at risk of developing Medication Related Osteonecrosis of the Jaw (MRONJ) – A primary prevention strategy. Saudi Pharm J. 2020;28(6):771-778.
Kün-Darbois JD, Fauvel F. Medication-related osteonecrosis and osteoradionecrosis of the jaws: Update and current management. Morphologie. 2021;105(349):170-187
Goker F, Grecchi E, Grecchi F, et al. Treatment of medication-related osteonecrosis of the jaw (MRONJ). A systematic review. Eur Rev Med Pharmacol Sci. 2021;25(6):2662-2673.

Ouazzani Touhami, Mohamed
Undergraduate student at the Complutense University of Madrid (UCM).
Ruiz Rincón, Miguel
Undergraduate student at the Complutense University of Madrid (UCM).
Benito López, Paula
Undergraduate student at the Complutense University of Madrid (UCM).
Bazal Bonelli, Santiago
Associate Lecturer on the Postgraduate Specialisation in Oral Surgery and Implantology, Faculty of Dentistry. Complutense University of Madrid (UCM).
Sánchez-Labrador, Luis
Honorary Associate Lecturer. Department of Clinical Dental Specialties. Complutense University of Madrid (UCM).
López-Quiles Martínez, Juan
Senior Lecturer. Director of the Postgraduate Specialisation in Oral Surgery and Implantology, Faculty of Dentistry, Complutense University of Madrid (UCM).