Clinical case
Published in spanish Científica Dental Vol. 17. Nº 3. 2020 www.cientificadental.es
Conservative approach in a patient with multiple radiolucent periapical lesions using endodontics and surgery
Case report of a 43- year old male patient with multiple periapical radiolucent lesions caused by endodontic failure in teeth supporting a metalloceramic prosthetic rehabilitation, who came to the clinic to assess the possibility of keeping his teeth.
After clinical and radiological examination with periapical x-rays and cone beam computer tomography (CBCT), we decided to use a combined endodontic-surgical approach.
Clinical evolution was favourable, and the radiographic and tomographic controls showed complete healing of the periapical radiolucent lesions.
Endodontic retreatment combined with periapical microsurgery are effective tools for conservative treatment of teeth with periapical lesions caused by endodontic failures.
A 43-year-old male patient, with no relevant medical history and prosthetic rehabilitation using intraradicular posts and metal-ceramic crowns from 16 to 26, who came to the clinic due to recurrent infections and fistulas in the anterosuperior sector and 25-26 zone. The patient had had the extraction of all of them proposed to him, with the placement of implants, but wanted to assess the possibility of keeping his teeth.
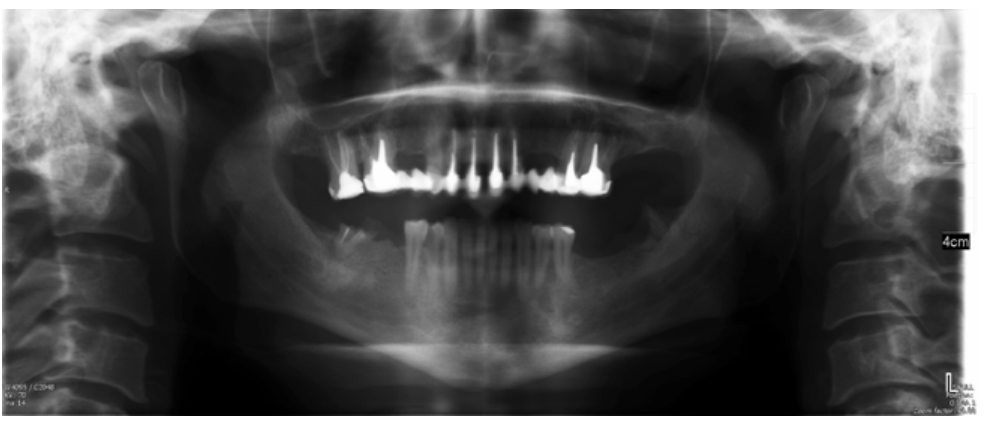
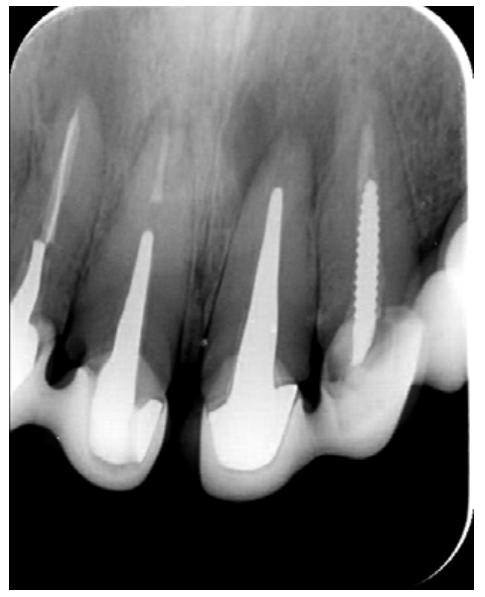
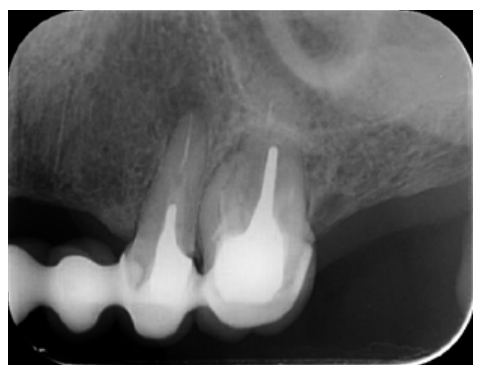
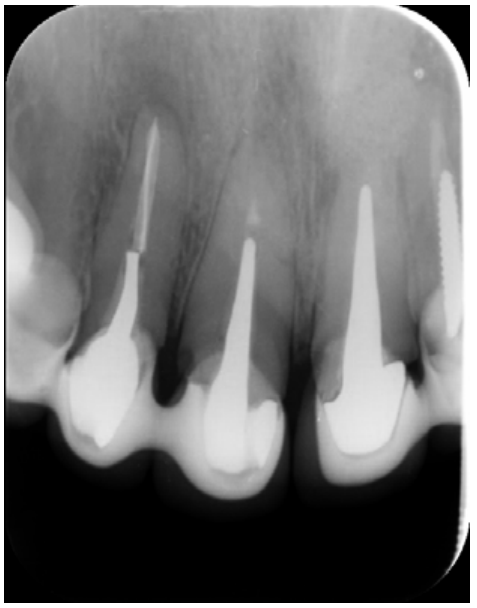
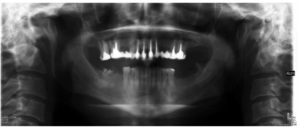
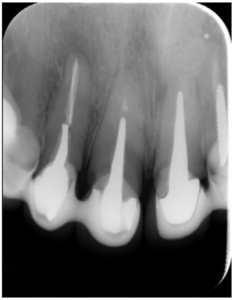
The patient provided an orthopantomography (OPG) as a radiological study (Figure 1). Periapical radiographs (Figures 2 and 3) were performed and a clinical examination including periodontal assessment of the affected teeth, without observing increased probing depths that could indicate the existence of endoperiodontal lesions.
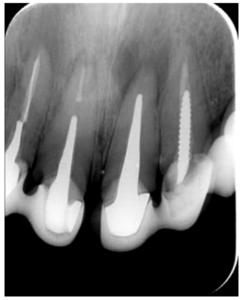
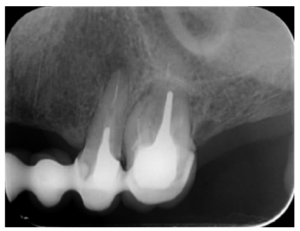
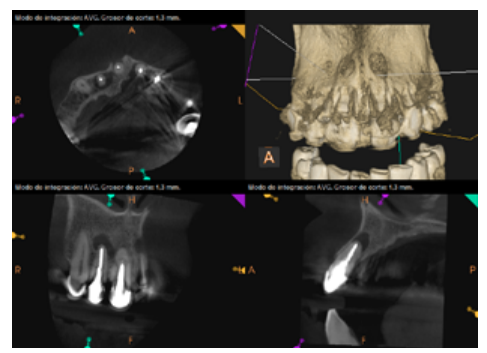
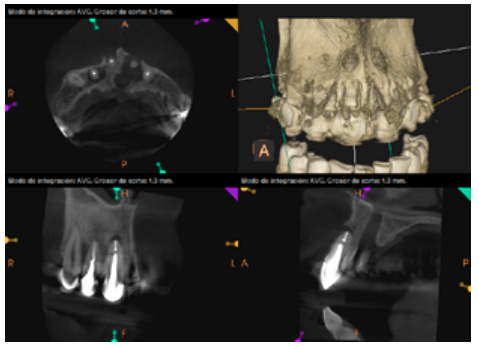
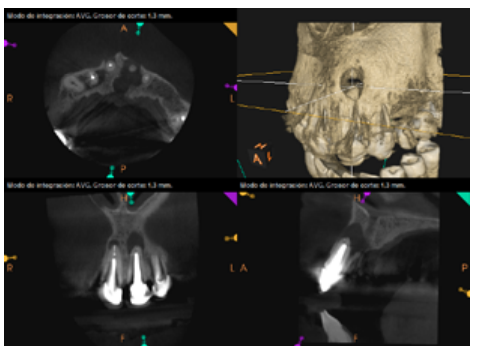
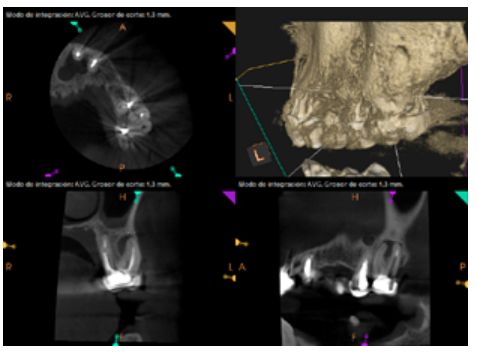
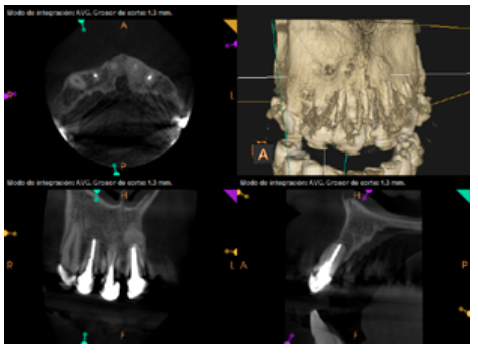
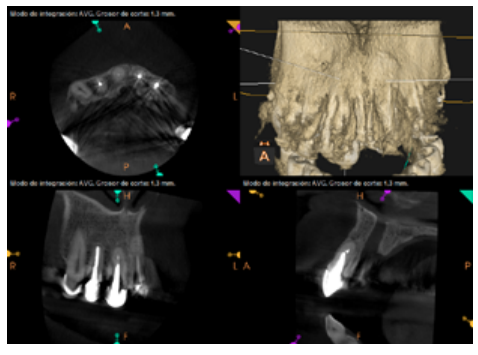
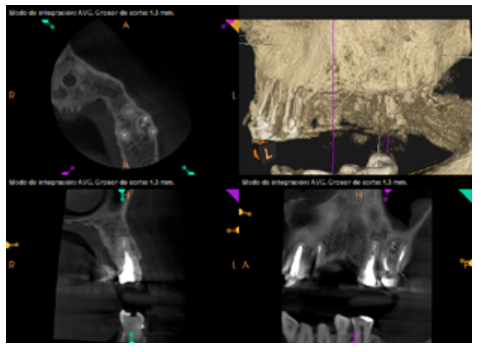
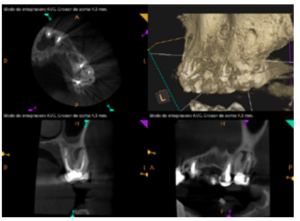
To confirm the endodontic origin and the size of the lesions, tomographic examinations were performed with a slice thickness of 75 microns using CBCT CS8100 (Carestream Dental™), in which radiolucent periapical lesions were observed at the level of 12, 11, 21 (with bicortical involvement), 25 and vestibular roots at 26 (Figures 4 to 8).
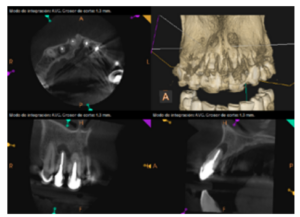
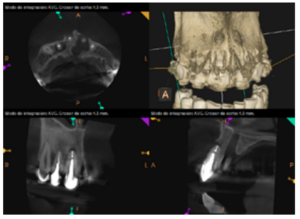
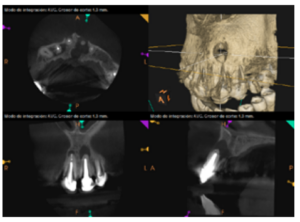
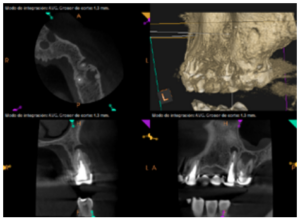
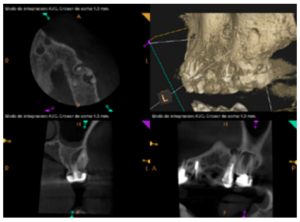
The patient was informed about his dental situation, and consent was obtained to perform apical microsurgery for three upper incisors (12, 11 and 21) and the need to use guided bone regeneration techniques (GBR) in 21.
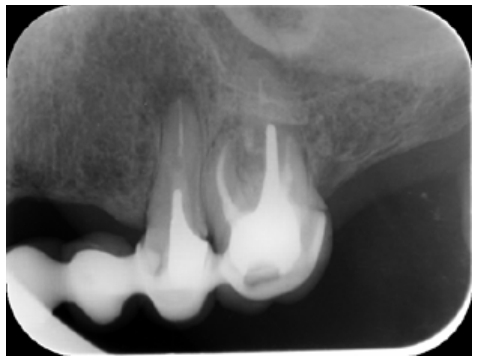
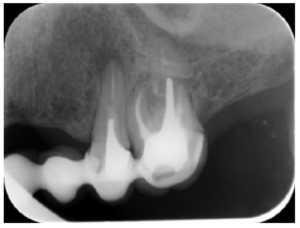
Meanwhile, the vestibular roots of 26 showed clearly deficient root canal treatment, being underextended by several millimetres, as well as an omitted mesiopalatine canal (MP). Therefore, the need to repair the root canal treatment was proposed to the patient before performing microsurgery on tooth 25 (Figure 9).
Periapical microsurgery was performed under magnification using an operating microscope (KapsTM) at the level of the upper incisors. Access to the apical lesions was achieved after a modified Neumann incision. Once these lesions were eliminated by dental excavation and curettage, apicectomies were performed, removing the last 3 mm of each root, and retrocavities 3 mm deep using ultrasound (Newtronc, Satelec) and obturation using bioceramic cement (Biodentine™, Septodont) were performed. In 21, a collagen membrane (Bioguide™, Geistlich) was placed in the palatal fundus and the defect was filled with porous bone matrix of bovine origin (BioOss™, Geistlich) before placing a new collagen membrane on the vestibular and suturing the flap using simple stitches with 5/0 Polyamide monofilament (Supramid™, Braun).
Subsequently, the canals of the buccal roots of 26 were retreated, performing a coronal access through the crown, eliminating part of the cast stump, locating the omitted MP canal and unobturating the mesiobuccal (MB) and distobuccal (DB). Once these canals were disinfected and shaped, they were filled with bioceramic sealing cement (BioRoot RCSTM, Septodont) and guttapercha.
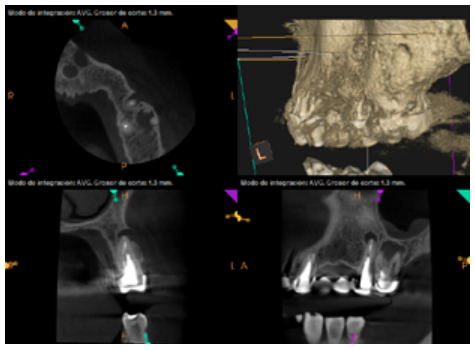
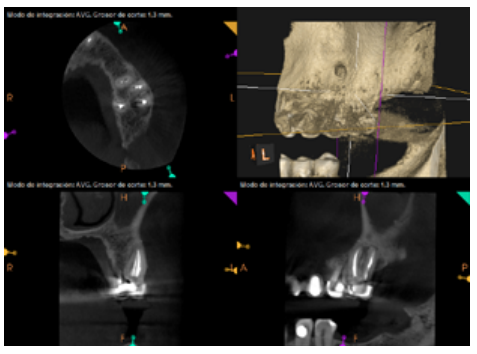
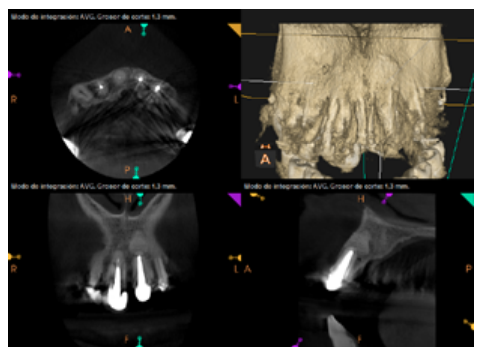
Six months later, the patient went for a check-up without any symptoms, both anteriorly and posteriorly. Periapical radiographs showed a decrease in the size of the pre-existing radiolucent periapical lesions (Figures 10 and 11). Given that the lesion on 25 remained to be treated, a control CBCT was performed where improvement at the level of 26 was verified (Figure 12), so microsurgery on 25 was scheduled.
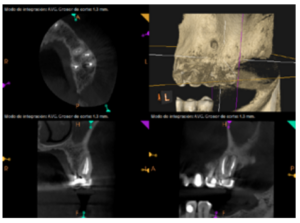
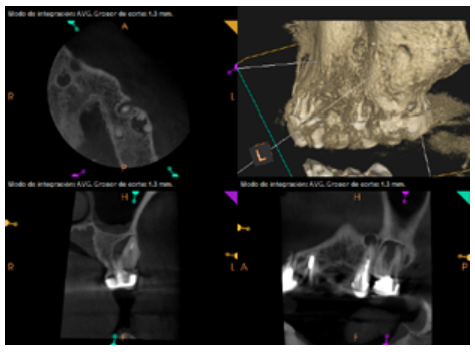
After the microsurgery was performed on 25, the patient had no symptoms and the control tomographies at 12 months (in 25) and at 18 months in the remaining teeth showed regeneration of pre-existing radiolucent periapical lesions on all treated teeth (Figures 13 to 16).
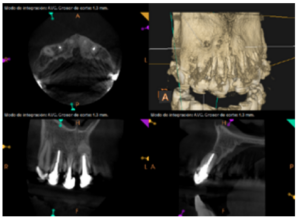
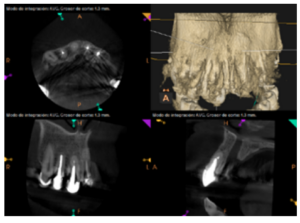
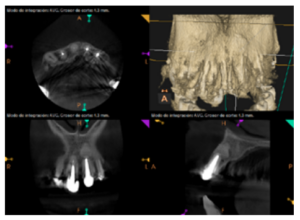
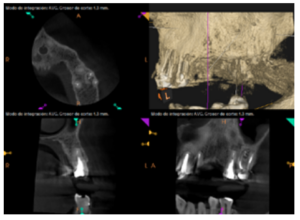
For proper diagnosis of radiolucent lesions at the periapical level, it is important to have radiological images that accurately show their location and size. In our case, the patient provided an orthopantomography; however, this type of radiography is not suitable for diagnosis in endodontics. The periapical radiography provides better definition, especially at the anterior level, as it is less distorted. CBCT is currently the most reliable radiological test, since it provides 3D images, which help to give a more accurate diagnosis1,2.
When there are teeth failures after numerous previous treatments, as in the case described, it is important to approach the diagnosis from a multidisciplinary point of view. Periodontal exploration by probing is essential to determine the periodontal status and rule out the presence of endoperiodontal lesions of periodontal origin, as well as vertical fractures3 .
Conservative therapeutic options for failures of endodontic origin are non-surgical root canal retreatment and periapical surgery. Various studies show similar success rates (around 75%) for both treatments, so other aspects should be considered before deciding which to follow; such as ease of access via the coronal approach and the quality of the root canal obturation from previous endodontic treatments4,5.
The intracanal posts in the single-root teeth in our case, whose removal would have entailed the sacrifice of the scarce remaining tooth that could remain under the metal ceramic crowns, made us opt for periapical surgery on these teeth. However, tooth 26 was underfilled by several millimetres in the buccal roots and the CBCT showed an omitted MP canal, so we opted for nonsurgical root canal retreatment in this tooth.
Authors such as Kim et al. highlight the importance of some aspects of the current surgical technique with respect to traditional periapical surgery, such as the performance of 3 mm apicoectomies without bevel, apical retropreparations with ultrasound and retrograde obturation with bioceramic materials; all this using the operating microscope, which is the fundamental tool that has greatly improved the prognosis of these treatments6 .
When performing combined endodontic-surgical treatment, one of the decisions to make is whether or not combined techniques of guided bone regeneration (GBR) should be applied7 .
As in other maxillo-mandibular bone defect reconstructions, we must know if the defect has a critical or non-critical size8,9. In the former, spontaneous regeneration will not occur in the patient while, in the latter, bone regeneration of the defect can be expected if the appropriate conditions, including the following, are met10
- Maintenance of the volume of the defect to be regenerated.
- Having a stable clot within this volume, which allows for its organisation and the migration of bone-forming cells.
- Preventing the invasion of fibroblasts and soft tissue surrounding the area from regenerating.
Another factor to take into account to determine the possibilities of regeneration of the periapical bone defect is the number of walls destroyed by the infectious process. The same degree of spontaneous recovery cannot be expected from multiwalled bone defects11, despite proper apical sealing and removal of associated inflammatory tissue.
In our case, we found different situations regarding periapical lesions, since they affected multiple teeth of different anatomies with different degrees of success in applying the aforementioned treatment.
In teeth 12, 11 and 25, the bone defect present was small (estimated at 0.2, 0.03 and 0.05 cm3 , respectively) with the absence of a wall. After the surgical approach, ostectomy with drilling bone in the apical area and curettage, it provided a favourable architecture for spontaneous regeneration; thus, the most reasonable attitude was not to provide biomaterials to try to improve bone regeneration.
However, the initial situation of piece 21, with a defect of 0.35 cm3 with two opposing walls of full vestibularpalatal thickness, which reached the nasopalatine vasculo-nervous pedicle without a break in continuity, suggested a different strategy.
In this type of bone defect, the cavity or residual space left after curettage of the apical granuloma tends to collapse more easily than those present at the level of 12, 11 and 25, where there is no possibility of invasion of fibroblasts from the palatal slope.
Therefore, after performing the apicoectomy and apical sealing of the ducts with BiodentineTM (Septodont), we applied additional GBR techniques to maintain this volume using resorbable collagen membranes (BioGide™, Geistlich) as a containment mechanism for the invasion of soft tissue on both the buccal and palatal slopes, associating the filling of the cavity with 0.5 g of porous bone matrix of bovine origin (Bio-Oss™, Geistlich) to prevent the collapse of the collagen membrane and to act as osteoconductive material12.
Radiological checks were carried out at 6, 12 and 18 months using CBCT, which showed the absence of symptoms and a reversal of the chronic infection, as well as progressivity and stability in apical bone regeneration. At the level of 21, periapical radiopacity was observed, without loss of volume, and an absence of invasion of the space preserved by the surrounding soft tissue. Although some authors have used plasma rich in growth factors (PRGF) associated with Bio-Oss™ and Bio-Gide™ in cases similar to ours, we obtained adequate results without using PRGF as an additional technique13.
The size of the rest of the periapical lesions led to favourable spontaneous regeneration, confirming that it is not necessary to perform GSR on small lesions that have no tunnel defect, as is the opinion of other authors7,14.
- Endodontic retreatment combined with periapical microsurgery are effective tools in the conservative treatment of periapical lesions of endodontic origin.
- Multidisciplinary diagnosis is essential to determine the most appropriate treatment in each case.
Diagnostic and therapeutic advances in the field of endodontics and periapical surgery allow a conservative approach to endodontic lesions, so teeth can be maintained and the bone, lost as a result of the lesions, can be recovered.
Ee J, Fayad MI, Johnson BR. Comparison of Endodontic Diagnosis and Treatment Planning Decisions Using Cone-beam Volumetric Tomography Versus Periapical Radiography. J Endod 2014;40(7):910–6.
Mota de Almeida FJ, Knutsson K, Flygare L. The impact of cone beam computed tomography on the choice of endodontic diagnosis. Int Endod J 2015;48(6):564–72.
Tsesis I, Rosen E, Tamse A, Taschieri S, Kfir A. Diagnosis of vertical root fractures in endodontically treated teeth based on clinical and radiographic indices: a systematic review. J Endod 2010;36(9):1455–8.
Torabinejad M, Corr R, Handysides R, Shabahang S. Outcomes of nonsurgical retreatment and endodontic surgery: a systematic review. J Endod 2009;35(7):930–7.
Arx von T, Peñarrocha M, Jensen S. Prognostic factors in apical surgery with root-end filling: a meta-analysis. J Endod 2010;36(6):957–73.
Kim S, Kratchman S. Modern endodontic surgery concepts and practice: a review. J Endod 2006;32(7):601–23.
Tsesis I, Rosen E, Tamse A, Taschieri S, Del Fabbro M. Effect of guided tissue regeneration on the outcome of surgical endodontic treatment: a systematic review and meta-analysis. J Endod 2011;37(8):1039–45.
Schmitz JP, Hollinger JO. The critical size defect as an experimental model for craniomandibulofacial nonunions. Clin Orthop Relat Res 1986;205:299-308.
Hollinger JO, Kleinschmidt JC. The critical size defect as an experimental model to test bone repair materials. J Craniofac Surg 1990;1(1):60–8.
Schenk RK, Buser D, Hardwick WR, Dahlin C. Healing pattern of bone regeneration in membrane-protected defects: a histologic study in the canine mandible. Int J Oral Maxillofac Implants 1994;9(1):13–29.
Goldman HM, Cohen DW. The Infrabony Pocket: Classification and Treatment. J Periodontol 1958;29(4):272–91.
Chiapasco M, Rossi A, Motta JJ, Crescentini M. Spontaneous bone regeneration after enucleation of large mandibular cysts: a radiographic computed analysis of 27 consecutive cases. YJOMS 2000;58(9):942-9.
Taschieri S, Rosano G, Weinstein T, Bortolin M, Del Fabbro M. Treatment of through-and-through bone lesion using autologous growth factors and xenogeneic bone graft: a case report. Oral Maxillofac Surg 2012;16(1):57–64.
Ochandiano Caicoya S. Relleno de cavidades óseas en cirugía maxilofacial con materiales aloplásticos. Rev Esp Cir Oral Maxilofac 2007; 29(1):21-32.

Grano de Oro Cordero, Eugenio C.
Degree in Dentistry, University Specialist in Endodontics, Private practice in Madrid.
Galán Hernández, Ramón
Doctor of Medicine and Surgery; Specialist in Oral and Maxillofacial Surgery; Ciudad Real University General Hospital; Private practices in Ciudad Real and Madrid.