Original article
Published in spanish Científica Dental Vol.17. nº3. 2020. www.cientificadental.es
Pilot clinical study of the efficacy of a hyaluronic acid (1%) and chlorhexidine (0.20%) gel in post-extraction dentistry
Objective: The objective of the present study was to compare the efficacy, in terms of oral healing and post-surgical pain, in a group of patients treated with an oral application of 1% hyaluronic acid (HA) together with 0.20% chlorhexidine (CHX), compared to patients treated with placebo and a third group treated with HA 0.20%, CHX 0.20% + Panthenol.
Method: The study design is an analytical, experimental, randomised, blind, prospective longitudinal study. A sample of 45 patients was randomised and divided into 3 comparison groups of 15, with each group receiving a different composition gel after a dental extraction. The control group received a gel of 0.20% hyaluronic acid and 0.20% chlorhexidine; the placebo group was applied a gel of similar consistency but without the active ingredients; and the experimental group received a gel with 1% hyaluronic acid and 0.20% chlorhexidine. Efficacy variables were measured at 24, 48 and 72 hours and 7 days.
Results: For post-operative pain, we found no statistically significant differences in any of the groups analysed. For healing, the group receiving 1% hyaluronic acid and 0.20% chlorhexidine had the best results from a statistical point of view in the first 24-48 hours compared to the other two groups.
Conclusions: The results obtained seemed to show that topical application of 1% hyaluronic acid together with 0.20% chlorhexidine influences soft tissue healing positively after a tooth extraction; however, it does not seem to have any beneficial effects on post-operative oral pain.
There are countless pharmacological chemicals used in dentistry for different purposes (e.g. for antiplaque, remineralisng, bleaching and desensitising) used in various forms (e.g. mouthwashes, gels and varnishes). There is scientific evidence about the role they play as adjuvants for various dental treatments applied in dentists to improve therapeutic response. The properties required of these therapeutic oral process adjuvants are specificity, efficiency, substantivity and safety.
Chlorhexidine is the antiplaque agent of choice and probably the most effective against gingivitis and reducing dental biofilm, both inhibiting its synthesis and preventing adhesion to teeth. The most common form it is present in mouthwashes is chlorhexidine digluconate. Based on the aforementioned properties of this compound, its activity in consultations is in a preventive, therapeutic and clinical sphere1-8.
Hyaluronic acid is a glycosaminoglycan consisting of glucuronic acid and N-acetylglucosamine. Its two most significant properties are as a lubricant and buffer due to the large concentration of water it can retain, conferring it extraordinary elasticity and acting as a defensive barrier in tissues. The clinical qualities of this product are based on improving tissue healing and promoting angiogenesis, and re-epithelialisation based on fibroblastic stimulation, increasing the production of growth factors and biosynthesis of various types of collagen. It is used in dentistry mostly in direct application gels for soft tissue lesions in oral cavities, where studies reveal a reduction in painful symptomatology in the first 24 hours of application9 . It is worth mentioning that there are few studies of hyaluronic acid applied in oral cavity soft tissues compared with other products used regularly in the mouthwash sector10-14. The objective of this study was to evaluate the effects of 1% hyaluronic acid/0.20% chlorhexidine to treat post-surgical pain and promote healing in dental alveoli.
This is an analytical, experimental, randomised, blind, prospective, longitudinal study carried out according to the Declaration of Helsinki. The San Carlos Clinical Hospital Ethical Clinical Research Committee approved the study with the Code 129RX and all patients were properly informed about it and had to read and sign an informed consent form before participating in the study.
The patient selection was randomised by the data sampling randomisation software, AleatorMetod.xls, into the following study groups:
- Experimental (1% hyaluronic acid and 0.20% chlorhexidine digluconate).
- Placebo.
- Control (0.2% hyaluronic acid, 0.20% chlorhexidine digluconate and panthenol).
All tubes containing the product applied in each group were white and coded with a number unknown to the operator. The relationship between the tube numbers and their content based on the groups was established once the study ended with the data collection notebook. The student was treated by the same operative at all times to prevent bias in the measurements.
Patients were those who attended a clinical consultation at the Centre for Advanced Studies in Implantology and Oral Surgery in Madrid, and were candidates for dental extraction in the positions and conditions required by the study. All data were recorded in a data collection notebook, including possible adverse effects.
The patient recruitment period was from February and August 2018. Patients were included in the study based on the inclusive criteria detailed below:
- Volunteers for the research project admitted after the explanation and signing of the written informed consent.
- Aged between 18-69 years of either sex.
- Teeth extracted: Groups 15-25 and 35¬45 due to decay or periodontal disease without active infection.
- People capable of understanding and carrying out the instructions explained by the principal investigator.
- Good physical condition, ASA I or II, not taking medication.
- Patients collaborating with the programmed appointments in the study.
The treatment to be performed was explained and specific informed consent given, especially regarding local anaesthesia and exodontics. Patients were also informed about the confidentiality of medical data and the procedure. Patients had the option of abandoning the study at any time.
All patients were informed of the inherent risks of dental extraction attached in this study. They were also informed about the application of the chlorhexidine and hyaluronic acid antiseptic products to be applied and the measurements taken.
The tooth extraction was performed in all patients following the inclusion criteria with the minimum surgical trauma by a single operator. To evaluate the degree of healing, the distance was measured in mm with a gauge at the time of extraction. The lingual or palatal vestibular median edge of the soft tissue of the alveolar process of the extracted tooth was taken as a measure and evaluated at baseline, then at intervals of 24h, 48h, 72h and a week. The operator applied the product assigned to the patient in a blind manner in the extraction area after the exodontic procedure, before explaining the need for the patients to apply it themselves at home 3 times a day after meals for 7 days. All patients received written basic post-extraction standards.
To record pain, patients received a data collection sheet with an analogue visual scale (see figure), according to severity: none, mild, moderate or intense. A total of 45 patients were recruited, with each study group containing 15. The sample size was not determined but, similarly to other research studies published in the scientific literature, groups of similar sizes were formed to evaluate the direction of the study target variables, using descriptive statistical parameters; and, as a result, to assess the interest in increasing the sample.
The Shapiro-Wilk goodness-of-fit test for the normal distribution was performed for the quantitative variables of healing and pain. Values that fitted the Gaussian distribution were obtained in both cases throughout the study, as well as for the calculated differences between the different periods; therefore, they are summarised with the mean and standard deviation.
The differences in healing and pain between treatments throughout the study were calculated with the onefactor ANOVA test. The differences between groups for 2 x 2 were carried out with the Bonferroni multiple comparison test.
The changes in healing and pain values over time were analysed with general linear model (GLM) repeated measures, as were the differences in the changes between the different treatments. These same analyses were calculated with variables resulting from the difference (delta) for each patient and for the different healing and pain measurement times (A = Initial valuefinazl value). Finally, the percentage difference in these values was calculated at all times with respect to initial values or decrease ratio (= (initial-final values) x 100/initial value), by studying the evolution of these variables also as GLM repeated measures, as well as the differences in evolution between the groups.
The difference values (A) and the ratios are summarised with the mean and the standard deviations for each case, calculating the 95% confidence intervals of the means.
A safety level 95% was considered, leading to a statistical significance of p<0.05.
All analyses were performed with the SPSS Statistical Package, version 24.0 (IBM Corp. Released 2016. IBM SPSS Statistics for Windows, Version 24.0. Armonk, NY: IBM Corp.)
Healing analysis by treatment groups (placebo, control and experimental)
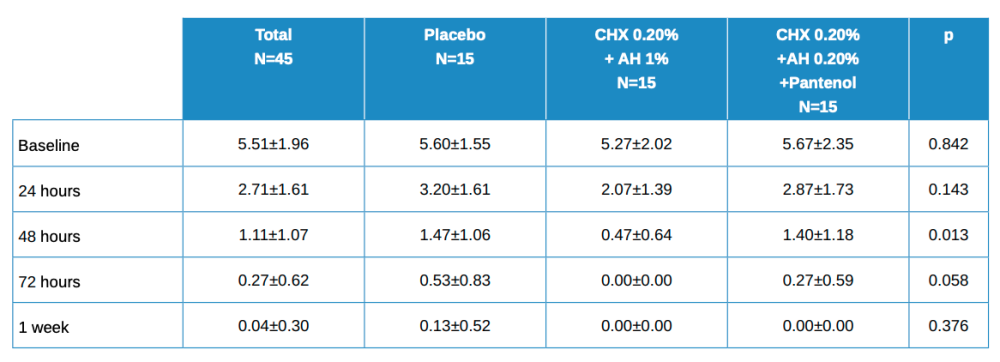
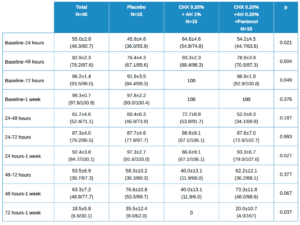
Table 1 shows the main descriptive data for the patients throughout the study as a total and by treatment group.
Baseline values are somewhat lower in the experimental group (AH 1%, CHX 0.20%), i.e. a little higher in the other two, although these differences were not statistically significant. In subsequent visits, the results were somewhat higher in the placebo group and lower for the other groups, although these differences were only statistically significant at 48 h.
The evolution in the global figure is statistically significant, with p<0.001. The differences in the evolution between the 3 treatment groups had a value of p=0.380, which was not statistically significant for any of the 2×2 comparisons between groups.
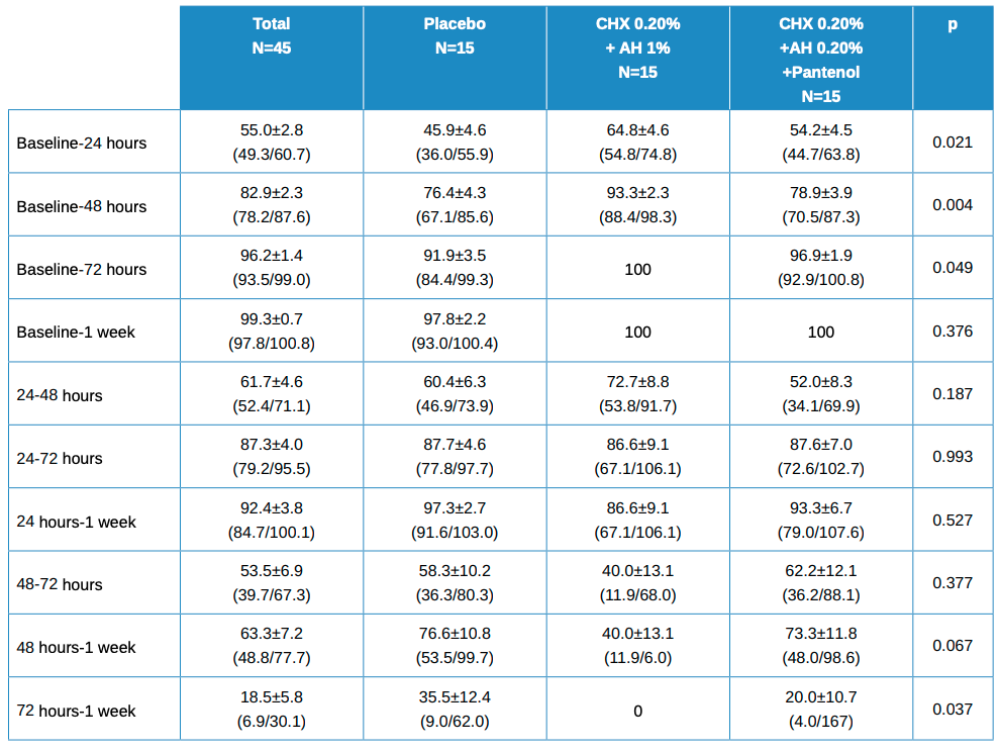
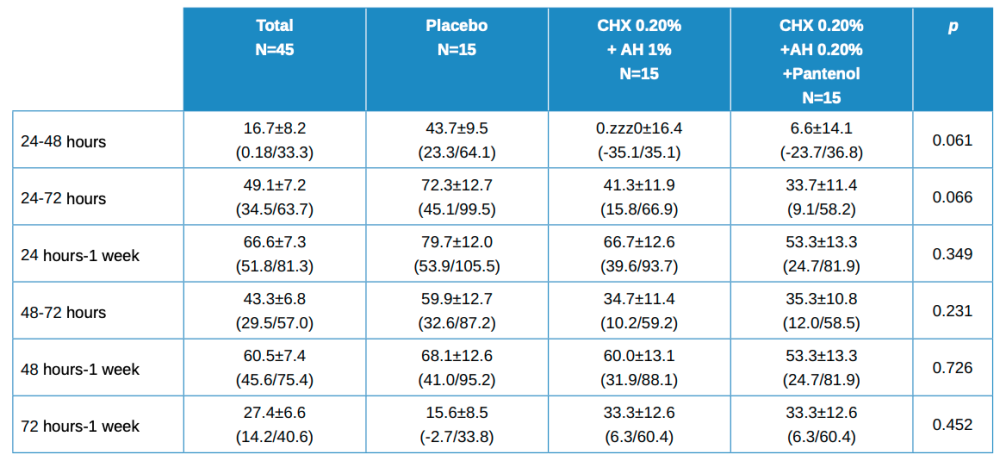
The values for the decrease ratio of these healing variables are shown in Table 2, with the mean ± standard deviation values, with the mean confidence intervals in brackets. Statistically significant differences were found in the differences between groups when the differences for each patient between baseline and 24, 48 and 72 hours were calculated. No statistically significant differences were found for other comparisons.
Statistical differences between treatment groups, 2 x 2, worth noting are:
- Baseline vs 24 hours in Placebo vs Test (HA 1%, CHX 0.20%; p=0.017.
- Baseline vs 48 hours in Placebo vs Test (HA 1%, CHX 0.20%); p=0.006.
- Baseline vs 48 hours in Test (HA 1%, CHX 0.20%) vs (HA 0.20%, CHX 0.20%, Panthenol); p=0.022.
- Baseline vs 72 hours in placebo vs Test (HA 1%, CHX 0.20%); p=0.047.
- 72 hours vs 1 week in Placebo vs Test (HA 1%, CHX 0.20%); p=0.033.
Analysis of pain in treatment groups
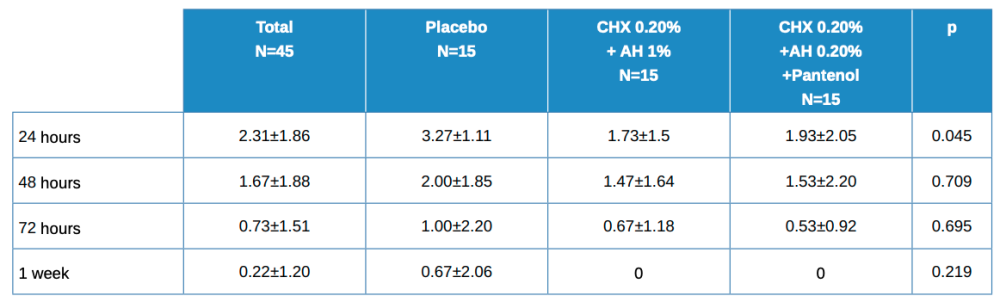
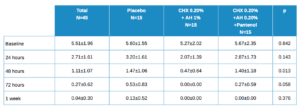
Table 3 shows the descriptive data of patients by total and by treatment type. The placebo value is higher for all time points, but statistically significant only at 24 hours. The evolution in the total is statistically significant with p<0.001. The differences in evolution among the 3 treatment groups has a statistically significant value of p = 0.425.
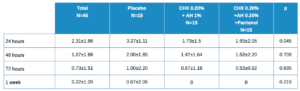
As with healing, the percentage decrease is calculated at different time points for pain. These values are shown in Table 4, with the mean ± SD with its confidence intervals in brackets. There are no statistically significant differences, although the values at 24-48h and 24-72h are close to this.
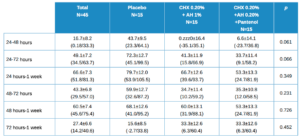
Values close to statistical significance between treatment groups, 2 x 2, worth noting are:
- 24 h vs 48 h in Placebo vs Test (HA 1%, CHX 0.20%); p=0.068
- 24 h vs 48 h in Control (HA 0.20%, CHX 0.20%, Panthenol) vs Test (HA 1%, CHX 0.20%); p=0.068
- 24 h vs 72 h in Control (HA 0.20%, CHX 0.20%, Panthenol) vs Test (HA 1%, CHX 0.20%); p=0.064.
Topical medication in the treatment of various oral processes is easy to apply. A notable aspect of this study is that the application of a gel (experimental, control or placebo) is that it seems to improve the perception of pain across the board. This is confirmed by Nolan and Lee14,15. Consulting the bibliography and confirmed by our study, the absence of side effects during the prescribed application period promotes continuation of the treatment14-16. The degree of healing results for the test composition (1% HA) were more favourable than the placebo or control (0.20% HA) groups. No confirmation of these data could be found in the literature as there are no similar studies applying the product on post-extraction alveoli, as it is mostly applied to ulcerated lesions of the oral mucosa. The physical barrier achieved after application of products in gel format probably makes it difficult for infections to appear, as authors such as Saxen17 and Porter18. found. Also, the properties of hyaluronic acid, such as water absorption and subsequent hydration, and structure, in the sense of being the main composition of the extracellular matrix14-17 seem to indicate a 1% concentration of high molecular weight hyaluronic acid (experimental group) may be more beneficial in terms of healing. Our study applied the products 3 times a day for 7 days, and studies consulted17-19 show that the dosage in relation to product application may be fundamental, since these authors found a decrease in the benefits of hyaluronic acid when the application changed from continuous to 3 times a day.
Based on the results obtained, applying a 1% hyaluronic acid and chlorhexidine digluconate gel has a beneficial effect for healing within the first 24-48 h of application, compared to the other groups.
We found a certain improvement in the perception of pain by the patient in observations made during the first 24 h. A number of results approached statistical significance for healing of the post-extraction alveolus; therefore, we should contemplate increasing the sample size as well as the dosing frequency, so that a clearer result could be obtained in future studies in terms of statistical power.
Addy M, Moran J, Newcombe R. A comparison of 0,12% and 0,1% chlorhexidine mouthrinses on the development of plague and gingivitis. Clin Prevent Dent 1991; 13: 26-9.
Addy M, Wade WG, Jenkins S, Gooldfield S. Comparison of two commercially available clorhexidine mouthrinses: I stainning and antimicrobial effects in vitro. Clin Prevent Dent 1989; 11: 10-4.
Banting D, Bosma M, Bollmer B. Clinical effectiveness of a 0,12% chlorhexidine mouthrines over two years. J Dent Res 1989; 68 (Spec. Issue): 1716-8.
Bascones A. Periodoncia Clínica e Implantología Oral. Madrid: Ediciones; Avances Médico-Dentales 2001; 455-71.
Twetman S. Antimicrobials in future caries control? A review with special reference to chlorhexidine treatment. Caries Res 2004;38: 223-229.
Yiu CK, Wei SH. Clinical efficacy of dentifrices in the control of calculus, plaque, and gingivitis. Quintessence Int 1993; 24: 181-188.
Quiryne M, Auontroodt P, Peeters W, Pauwels M, Couche W, Van Steenberghe. Effect of different clorhexidine formulations in mouthrinses on de novo plaque formation. J Clin Periodontol 2001; 1127-36.
Ragno JR, Szkntwik AJ. Evaluation of 0,12% chlorhexidine rinse on the prevention of alveolar osteitis. Oral Surg Oral Med Oral Pathol 1991; 72: 524-26
Rabasseda X. Ácido hialurónico. Papel terapéutico en la gingivitis. Drugs Today 1997; 6: 1-21.
Gontiya G, Galgali SR. Effect of hyaluronan on periodontitis: a clinical and histological study. J Indian Soc Periodontol 2012; 16 (2): 184-192.
Kosaki R1, Watanabe K, Yamaguchi Y. Overproduction of hyaluronan by expression of the hyaluronan synthase Has2 enhances anchorage-independent growth and tumorigenicity. Cancer Res 1999; 59 (5): 1141-1145.
Mesa FL, Gijón J, Cabrera A, López C, O’Valle FJ. Efecto de un gel de ácido hialurónico en la enfermedad periodontal. Estudio clínico e histopatológico. J Periodoncia 2001; 11 (2): 107-116.
Paraskevas S. Randomized controlled clinical trials on agents used for chemical plaque control. Int J Dent Hyg 2005; 3: 162-178.
Nolan A, Ballie C, Badminton J, Rudralingham M, Sey mour RA. The efficacy of topical hyaluronic acid in the management of recurrent aphthous ulceration. J Oral Pathol Med 2006; 35 (8): 461-5.
Lee JH, Jung JY, Bang D. The efficacy of topical 0,2% hyaluronic acid gel on recurrent oral ulcers: comparison between recurrent aphthous ulcers and the oral ulcers of Behçet´s disease. J Eur Acad Dermatol Venereol 2008; 22 (5): 590-5.
Nolan A, Badminton J, Maguire J, Sey mour RA. The efficacy of topical hyaluronic acid in the management of lichen planus. J Oral Pathol Med 2009; 38: 299- 303.
Saxen MA, Ambrosius WT, Rhemtula al – KF, Rusel AL, Eckert GJ. Sustained relief of oral aphthous ulcer pain from topical diclofenaco in hialuronan: a randomized, doublé blind clinical trial. Oral Surg Oral Med Oral Pathol Oral Radiol Endod 1997; 84 (4): 356-61.
Porter S. Transient benefits for topical hyaluronic acid in recurrent aphthous ulceration. J Oral Pathol Med 2006; 35(8): 461-5.
Cantor JO, Nadkarini PP. Hyaluronan: The Jekyll and Hyde molecule. Inflamm Allergy Drug Targets 2006; 5: 257-60.

Ripollés de Ramón, Jorge
Doctor in Dentistry, Madrid Complutense University (UCM); Master’s Oral Surgery, Implants and Periodontics, Coruña University (UC).
Serrano Sánchez, Víctor
Degree in Dentistry (UCM). Master’s in Dental Sciences (UCM); Expert in Periodontics (UCM); studying Master’s in Periodontics and Implants (UCM).
Colmenero Ruiz, Constantino
Dentistry at UCM; Magister in Oral Surgery Príncipe de Asturias University Hospital-Alcalá University (HUPA-UAH); Magister in Orthodontics, Alcalá University (UAH).
Vaello Checa, Iris
Degree in dentistry; Master’s in Dental Sciences (UCM); studying Master’s in Orthodontics (UCM).