Original Article
Published in spanish Científica Dental Vol. 18. Nº 3. 2021 www.cientificadental.es
Pilot study on the diagnosis of and factors related to hyposialia in patients with xerostomia at a university clinic
Xerostomia is a subjective sensation of dry mouth that may or may not be accompanied by a decrease in the amount of saliva. Hyposialia is a reduction in salivary flow, as measured by sialometry. The aims of the study were to establish the total percentage of patients with actual reduced saliva flow (hyposialia) in a group of patients with perceived reduced saliva flow (xerostomia) and analyse the differences between patients with xerostomia associated with hyposalia and patients with subjective xerostomia.
28 patients with xerostomia were part of the study between November and March 2020-2021 at the Polyclinic of the European University of Madrid. A comprehensive medical history was prepared, 3 questionnaires were completed (Xerostomia Inventory, Perceived Stress Scale and OHIP- 14) and unstimulated sialometry was performed for 5 minutes. Data analysis was performed with the Stata IC v 14 statistics program.
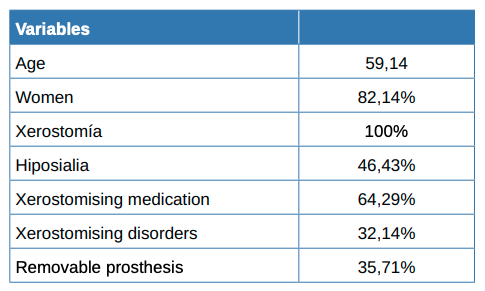
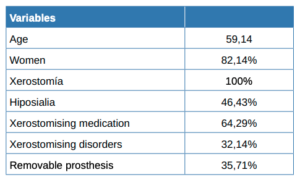
82% of the total patients who reported dry mouth were women, with a mean age of 59.14 years. Less than half of the patients (46%) had hyposialia as evidenced by sialometry. There were more patients with dental prostheses in the group suffering from hyposialia compared to the group with normal salivary flow. Both groups showed a similar number of xerostomising disorders and drugs. There were no significant differences between either group regarding the completed questionnaires.
Xerostomia is the subjective sensation of a dry mouth; whereas hyposalivation is an objectively measured lower volume of saliva produced, according to accepted, standardised values. These two conditions are often confused and misused; they may complementary to each other, but not always. This pathology affects speech, chewing, swallowing and general status. It is also uncomfortable for wearers of prostheses; increases the incidence of tooth decay and periodontal disease; changes the taste of food; leads to halitosis and other symptoms that greatly affect the quality of a patient’s life1 . The literature establishes the prevalence of xerostomia at around 20% of the population, although studies that place it in a range of 10-46% have been published2 . Among such patients, 30% are women and there is a higher percentage of those with advanced age2 . A study conducted in an Australian elderly population found 1 in 5 had xerostomia or hyposalivation, with 1 in 6 having both pathologies: 5.6% of the total sample3 .
Given the known aetiology of xerostomia and hyposalivation, there are many studies that focus on the high percentage of the elderly with this pathology due to their polymedicated status, as this is a risk factor for the change in the composition of saliva, leaving aside any general problems of ageing4 . Among the 14 firstlevel medication groups of the Anatomical Therapeutic Chemical (ATC) classification system, 9 were reported as xerostomising medications. The most common are anticholinergics, antidepressants, antihistamines, antiParkinsonians, anti-hypertensive and sedative agents such as benzodiazepines. All of them are very common drugs in the clinical histories of people of all ages, not just the elderly5 .
Many common diseases have xerostomia among their symptoms, such as diabetes or depression, with this symptom getting worse with increasing prescribed medication, as is the case with Sjogren’s syndrome and uncontrolled Parkinson’s6-8. Even certain treatments, such as head and neck radiation, have this type of side effect in most patients who receive it9,10.
Stress is a risk factor for xerostomia; it is evaluated with questionnaires and is considered related to it11. Smoking also plays a crucial role in this pathology, where it thickens the texture of saliva instead of reducing its volume. The effects of smoking are dependent on the amount consumed12.
As these two pathologies are different, they have to be diagnosed differently. Xerostomia is a subjective disorder, and is evaluated by a questionnaire, of which here are several in the literature. Predominantly used is the xerostomia inventory, which contains 11 questions and gives a maximum score of 55. It is written in the first person: e.g. “I drink liquids to swallow the food”, “My eyes are so dry”13. Another simple diagnostic method to evaluate clinical signs is the Clinical Oral Dryness Score (CODS). This evaluates several parameters in a scoring scale of 10; among them are if the dental mirror adheres to the tongue, if there is saliva on the floor of the mouth and if there is a loss of papillae in the tongue14. However, to test for hyposalivation, an objective salivary flow measurement, sialometry, is required by. This is a simple test in which the patient expectorates into a container for an average of 5 minutes. This can be unstimulated sialometry or stimulated, using a sugar-free lemon sweet or chewing gum, for example15. Normal values for unstimulated sialometry are greater than or equal to 0.1 mL/min, and 0.7mL/min for stimulated16,17.
Currently, there are few effective treatments available. Initially, a change of habits; stress control; stopping or reducing smoking; reducing the dose of medication or replacing it with another less xerostomising; proper hydration; and eating acidic sweets or chewing gum to stimulate the glands18. Another more palliative treatment option is to use topical sialagogues, such as 1% malic acid, which has been shown to significantly increase saliva volume19. Systemic sialagogues, such as pilocarpine and cevimeline, parasympathomimetic and muscarinic agonists, have proven effective in the relief of hyposialia even in extreme cases such as patients receiving head and neck radiation. The disadvantages they have are that the stimulation duration is an average of two hours, and numerous side effects can appear20.
Given the confusion that exists between xerostomy and hyposalivation, and considering that they are not always linked and are managed differently in the clinic, the objectives of this study were to determine the proportion of xerostomic patients actually with hyposialia; the frequency of the disease in the different age groups and their distribution by sex. Their association with habits, stress levels, presence of xerostomising disorders and medications was also assessed.
The protocol for this observational, prospective and cross-sectional study was approved by the European University of Madrid Ethics Committee (Code CIPI/20/123).
All patients of legal age who attended the University Polyclinic of the European University of Madrid between November 2020 and March 2021 who answered in the affirmative to the question “Does your mouth feel dry?” were included. They were provided with a detailed verbal and written explanation of the study, before signing an informed consent to participation in it.
Firstly, an exhaustive medical history investigation was carried out. Then, 3 questionnaires were filled in: the Xerostomia Inventory, Perceived Stress Scale and OHIP-14.
Finally, unstimulated sialometry over 5 minutes was performed.
All patients were treated by the same investigator between 8 am and 1 pm in the Polyclinic of the European University.
The following variables were recorded for each patient: age, sex, consumption of alcohol, tobacco and other drugs, systemic diseases, habitual medication, dental prostheses held.
In the statistical analysis, absolute and relative frequencies were used to express the qualitative variables. As for the quantitative variables, the standard deviation and deviation were calculated in those that followed a normal or median distribution, and the interquartile range for those who did not. The proportion of patients with both xerostomy and hyposialia and respective 95% confidence intervals were recorded.
A chi square (or Fisher exact test) was performed to compare the qualitative variables of sociodemographic features, habits, comorbidity, usual medication, oral health status, quality of life and the perceived stress of patients, with or without hyposialia. A Student t-test (or Mann-Whitney U test), was performed for quantitative variables for independent samples. Statistical significance was considered a p-value of less than 5%. The Stata IC V.14 statistical package was used for data analysis.
Included in the trial were 28 participants with a subjective sensation of dry mouth (xerostomia), of whom 23 were women (82.14%) and 5 men. The mean age was 59.14 years (SD = 14.29), with the youngest being 27 and the oldest 79.
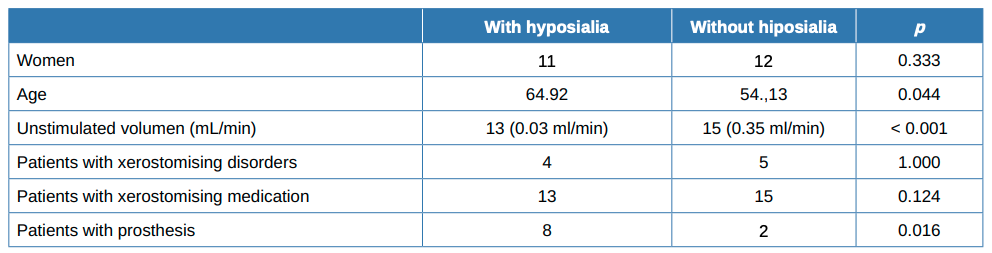
Almost half (13, 46.43%) of the 28 patients had hyposialia, measured via unstimulated sialometry, while 15 had symptomatic xerostomia without hyposialia. The median [Q1, Q3] unstimulated volume was significantly lower in the patients with hyposialia (objective xerostomia) than those with xerostomia without hyposialia (0.01 [0-0.04] vs. 0.22 [0.2- 0.4], mL/ min respectively; p <0.001).
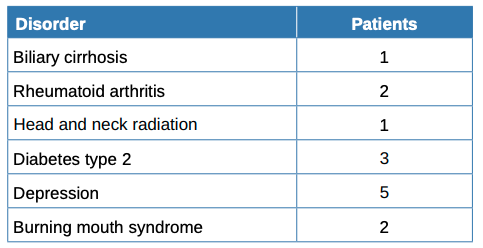
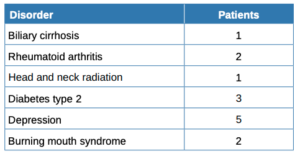
Table 1 summarises the sociodemographic and clinical features and habits of the patients while Table 2 compares patients with and without hyposialia. Patients with hyposialia were significantly older (64 ± 9.7 years vs 54.1 ± 15.9 years; respectively, p = 0.044). The number of xerostomising disorders was similar in both groups, with depression being the most frequent in all patients (Table 3). Selective serotonin reuptake inhibitor (SSRIs) antidepressants were the most common medication with a reported dry mouth side effect.
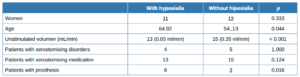
Measured habits were not significantly different between patients with and without hyposialia. No patient drank alcohol continuously or repeatedly, many occasionally or sporadically in company and some not atall. No patient reported consuming illegal drugs. Only 8 of the patients smoked, represent similarly across both groups. There was also no significant difference between patients with and without hyposialia regarding the amount of fluids drank per day, as well as in the oral hygiene habit of daily teeth brushing.
Finally, a greater proportion of patients in the hyposialia group had prostheses (61.5% vs. 13.3%, respectively; p = 0.016) as well as having fewer remaining teeth.
In this study, all patients responded to the question: Do you feel your mouth is dry? If they answered ‘yes’, this subjective sensation was enough for direct inclusion as a xerostomia patient1 . Of the 28 patients included in the study, 82% were women, who were more represented in the literature for xerostomia3,11. In a study conducted on 3,313 Swedish people, the percentage of nonmedicated female subjects with xerostomia was 18.8% compared to 14.6% of men. Similarly, the percentage of medicated female subjects with xerostomia was 32.5% compared to 28.4% of men who had the subjective sensation of a dry mouth21. This pathology is related to menopausal women on many occasions. The salivary glands contain sex hormone receptors, with the level of oestrogen being capable of varying the secretion and composition of saliva22. There are many studies that have focused on this without reaching very conclusive results: Minicucci et al.23 studied the volume of saliva in a group of women of menopausal age and compared them with a control group of women of childbearing age. There was a significant difference only in the volume of saliva in stimulated sialometry, not at rest. Eliasson et al.24 showed an increase in the volume produced by the minor salivary glands after a year of treatment with a weak oestrogen (oestriol) in women with more than 5 years of amenorrhea, improving the sensation of dry mouth. However, they showed a statistically significant increase only in stimulated saliva, not at rest.
The average age in this study was 59.14 years old, and older in the hyposialia group. In the aforementioned study, patients older than 60 years showed an exponentially increasing percentage in relation to age21. This increase in xerostomia with age has been reported in numerous publications3,4,11. The dilemma is to determine whether this relationship of age with xerostomia has its oetiological origin in age per se or in the increased number of drugs and disease associated with age. Nederfors et al.21 reported a low correlation between dry mouth symptoms in nonmedicated patients, thus reinforcing the xerostomia hypothesis as a secondary effect of medications or polymedication and not of age per se. However, Yehl et al.25 demonstrated a decrease in the total volume of saliva at rest in a cross -sectional study of 1006 patients, with the secreted stimulated by the parotid, and unstimulated and stimulated from the submandibular and sublingual, according to the age of the study group. 46% of the patients in the study who reported having a subjective sensation of a dry mouth had a sialometric volume of less than 0.1mL/min, and were thus hyposialic. This leads to the conclusion that the perception of dry mouth in a higher percentage of patients with xerostomia was subjective. This percentage is greater than that reported by Thomson et al.3 of 22.1% hyposialia, 20.5% xerostomia and 5.6% subjects who met both conditions. Notably, the patients with hyposialia (or “objective xerostomia”) had more removable prostheses, of whichever number or type, than patients with just xerostomia. The effect of xerostomia has been seen in the use of removable prostheses, and not of dentures, in the increase in the sensation of dry mouth 26. However, Gabay et al.27 defended saliva production stimulation through the use of a complete prosthesis, which increased the saliva volume by more than double a year after wearing them. Years later, Wolff et al.28 did not reach the same conclusion: they found the volume of saliva secreted from inserting the removable prosthesis increased after 2 days but, after 3 months, the volume was the same as in the first measurement. In this study, it was found that those patients with fewer remaining teeth were had hyposialia, which is directly related to the use of dental prostheses.
Before the sialometry, the 28 subjects in this study filled in 3 questionnaires: Xerostomia Inventory, OHIP-14 and Perceived Stress Scale; the latter was to determine the patient’s stress levels in the last month. The average score for the latter was 25.7 out of 56, with no significant difference seen between the groups. However, the literature has several studies reporting the relationship of stress and xerostomia11,29. Also, depression as stress is also a significant risk factor for xerostomia. The most common xerostomising disorder among the study patients was depression, with antidepressants in the xerostomising drug group. There were no differences in the consumption of xerostomising medication, and no increase in the pathology with the number of drugs; this was confirmed in these studies21,30,31. In a study carried out on a geriatric population, 44% of the medication prescribed to patients had hyposialia as a side effect, presenting with a greater number of drugs taken by women30. In the aforementioned Nederfors et al. study21, 32.1% of medicated patients had xerostomia, compared to 16.9% for the non -medicated group. Also, a linear relationship was found for the association between the sensation of a dry mouth and the total number of drugs consumed daily. The drugs that induce dysfunction of the salivary glands act directly on the central and peripheral nervous system, many of them dose-dependent, thus increasing severity31. Drugs are a clear significant risk factor for xerostomia, which increases with their number and dosage. We must assume that we have not obtained conclusive data in this field due to the small sample number.
There were no significant differences between the groups for the Xerostomia inventory results, with an affirmative average of 31.89. Taking into account that all participants were selected because they felt they had a dry mouth, inventory xerostomia can be assumed to be a good diagnostic tool. It uses simple vocabulary, with short and direct phrases; is easy to for patients to understand and respond to. However, the same could not be said for the Perceived Stress Scale. Given the advanced age and perhaps socioeconomic level of patients who come to university clinics for treatment, understanding the questions was difficult. All were very extensive, repetitive and complex when changing affirmation of the question to a denial. In general, after the initial questions, they were observed to lose interest and answered randomly. A simpler questionnaire for this purpose would be useful in the future.
There are many studies In the literature that use Xerostomia Inventory with very positive results; having been translated into numerous languages13,30.
OHIP-14 was similar, it being observed that most patients did not see their quality of life-threatened by the state of their oral health; there were no significant differences between the two groups. Marjolein et al.32 used these three questionnaires in their study of 114 patients. They found stress levels were directly associated with the OHIP-14 results, but with no statistically significant association being found between stress and saliva volume. This confirms the above observation, with the need to increase our study sample size to obtain conclusive data.
Given the SARS-COV2 pandemic, there were few patients able to participate in the study. Due to the fear of infection at a university clinic, patients were treated in boxes in large rooms, with a false feeling of insecurity. Also, dentistry is a practice where the patient cannot use a mask, which contributed to that perception of danger. Also, this serious crisis caused significant professional and economic instability, giving rise to a notable decrease in the number of patients, making our study one with a non-representative sample.
Another constraint on the number of participants was the time restriction for the study. The sialometry had to be done in the morning, in accordance with the circadian rhythm. Salivary glands function according to a genetic clock which varies with the time of day32,33. This further limited the testing time and ruled out all those patients who had to work in the morning.
Suggestions for future projects related to this or to its expansion would be a multicentre study with several investigators collecting data and thus increasing the sample size. The method would still be the same, but we suggest looking for a simpler alternative to the Perceived Stress Scale for understanding and execution of the questionnaire.
At the end of the study, more than half of the sample proved to have xerostomia which is the subjective feeling of a dry mouth, not confirmed by saliva testing, as is hyposialia. After studying the data collected, It was observed that those patients suffering from xerostomia were mostly elderly women. The group of hyposialic patients was notable for the proportion of people with dental prostheses. There was no significant differences between these 2 groups for xerostomising conditions or medication. Similarly, no significant differences were found between the groups in stress levels and the quality of life, as measured by the OHIP-14 and Perceived Stress Scale questionnaires.
To the Company, DENTAID, for providing us with the Xeros™ range of products for the study patients, as well as related bibliography.
To Dr Cristina Andreu- Vázquez, for her patience and help with all the methodology.
Navazesh M, Christensen CM, Brightman VJ. Clinical criteria for the diagnosis of salivary gland hypofunction. J Dent Res 1992; 71: 1363–9.
Hopcraft MS, Tan C. Xerostomia: an update for clinicians. Aust Dent J 2010;55(3):238-44.
Thomson WM, Chalmers JM, Spencer AJ, Ketabi M. The occurrence of xerostomia and salivary gland hypofunction in a population-based sample of older South Australians. Spec Care Dent 1999;19:20–3.
Dodds MW, Johnson DA, Yeh CK. Health benefits of saliva: a review. J Dent 2005;33(3):223–33.
Wolff A, Joshi RK, Ekström J, y cols. A guide to medications inducing salivary gland dysfunction, xerostomia, and subjective sialorrhea: A systematic review sponsored by the World Workshop on Oral Medicine VI. Drugs R D 2017;17(1):1-28.
Vasconcelos AC, Soares MS, Almeida PC, Soares TC. Comparative study of the concentration of salivary and blood glucose in type 2 diabetic patients. J Oral Sci 2010;52(2):293-8.
Anttila SS, Knuuttila ML, Sakki TK. Depressive symptoms as an underlying factor of the sensation of dry mouth. Psychosom Med 1998;60:215-8
Cersósimo MG, Tumilasci OR, Raina GB, y cols. Hyposialorrhea as an early manifestation of Parkinson disease. Auton Neurosci 2009;150:150–1.
Mercadante V, Al Hamad A, Lodi G, Porter S, Fedele S. Interventions for the management of radiotherapyinduced xerostomia and hyposalivation: A systematic review and meta-analysis. Oral Oncol 2017;66:64-74.
Ferlay J, Soerjomataram I, Dikshit R, y cols. Cancer incidence and mortality worldwide: sources, methods and major patterns in GLOBOCAN 2012. Int J Cancer 2015 1;136:E359-86.
Bergdahl M, Bergdahl J. Low unstimulated salivary flow and subjective oral dryness: association with medication, anxiety, depression, and stress. J Dent Res 2000;79:1652-8.
Petrušić N, Posavac M, Sabol I, MravakStipetić M. The effect of tobacco smoking on salivation. Acta Stomatol Croat 2015;49:309-15.
Thomson WM, Chalmers JM, Spencer AJ, Williams SM. The Xerostomia Inventory: a multi-item approach to measuring dry mouth. Community Dent Health 1999; 16: 12– 17.
Osailan SM, Pramanik R, Shirlaw P, Proctor GB, Challacombe SJ. Clinical assessment of oral dryness: development of a scoring system related to salivary flow and mucosal wetness. Oral Surg Oral Med Oral Pathol Oral Radiol 2012;114:597-603.
Navazesh M, Kumar SK. Measuring salivary flow: challenges and opportunities. J Am Dent Assoc 2008; 139 Suppl: 35S– 40S.
Navazesh M, Christensen CM, Brightman VJ. Clinical criteria for the diagnosis of salivary gland hypofunction. J Dent Res 1992; 71: 1363– 9.
Ship J, Fox PC, Baum BJ. How much saliva is enough? ‘Normal’ function defined. J Am Dent Assoc 1991; 122: 63– 9.
Visvanathan V, Nix P. Managing the patient presenting with xerostomia: a review. Int J Clin Pract 2010; 64:404-7.
Gómez-Moreno G, Cabrera-Ayala M, Aguilar-Salvatierra A, y cols. Evaluation of the efficacy of a topical sialogogue spray containing malic acid 1% in elderly people with xerostomia: a double-blind, randomized clinical trial. Gerodontology 2014; 31:274-80.
LeVeque FG, Montgomery M, Potter D, y cols., A multicenter, randomized, doubleblind, placebo-controlled, dose-titration study of oral pilocarpine for treatment of radiation-induced xerostomia in head and neck cancer patients. J Clin Oncol 1993;11:1124-31.
Nederfors MW, Isaksson R, Mornstad H, Dahlof C. Prevalence of perceived symptoms of dry mouth in an adult Swedish population- relation to age, sex and pharmacotherapy. Commun Dent Oral Epidemiol 1997; 25:211-6.
Välimaa H, Savolainen S, Soukka T, y cols. Estrogen receptor-beta is the predominant estrogen receptor subtype in human oral epithelium and salivary glands. J Endocrinol 2004;180:55-62.
Minicucci EM, Pires RB, Vieira RA, Miot HA, Sposto MR. Assessing the impact of menopause on salivary flow and xerostomia. Aust Dent J 2013;58:230-4.
Eliasson L, Carlén A, Laine M, Birkhed D. Minor gland and whole saliva in postmenopausal women using a low potency oestrogen (oestriol). Arch Oral Biol 2003;48:511-7.
Yeh C-K, Johnson DA, Dodds MWJ. Impact of aging on human salivary gland function: a community-based study. Aging: Clinical and Experimental Research 1998;10:421–8.
Tanaka A, Kellesarian SV, Arany S. Xerostomia and patients’ satisfaction with removable denture performance: systematic review. Quintessence Int 2021;52:46-55.
Gabay EL. Flow rate, sodium and potassium concentration in mixed saliva of complete denture-wearers. J Oral Rehabil 1980;7:435-43.
Wolff A, Ofer S, Raviv M, Helft M, Cardash HS. The flow rate of whole and submandibular/sublingual gland saliva in patients receiving replacement complete dentures. J Oral Rehabil 2004;31:340-3.
Bulthuis MS, Jan Jager DH, Brand HS. Relationship among perceived stress, xerostomia, and salivary flow rate in patients visiting a saliva clinic. Clin Oral Investig 2018;22:3121-3127.
Van der Putten GJ, Brand HS, Schols JM, de Baat C. The diagnostic suitability of a xerostomia questionnaire and the association between xerostomia, hyposalivation and medication use in a group of nursing home residents. Clin Oral Investig 2011;15:185-92.
Villa A, Wolff A, Narayana N, y cols. World Workshop on Oral Medicine VI: a systematic review of medication-induced salivary gland dysfunction. Oral Dis 2016;22:365-82.
Dawes C. Circadian rhythms in human salivary flow rate and composition. J Physiol 1972;220(3):529-45.
Papagerakis S, Zheng L, Schnell S, y cols. The circadian clock in oral health and diseases. J Dent Res 2014;93(1):27- 35.

Medina López-Chicheri, Paula
Master’s in advanced periodontics, Madrid European University, PhD student, Lecturer in the Clinical Dentistry Department of the Faculty of Biomedical Sciences, Madrid European University
Muñoz Corcuera, Marta
Doctor in Dentistry; Lecturer in the Clinical Dentistry Department of the Faculty of Biomedical Sciences, Madrid European University.
Navarrete Marabini, Natalia
Doctor in Dentistry; Lecturer in the Clinical Dentistry Department of the Faculty of Biomedical Sciences, Madrid European University.
Gil-Abando Lozano, Gabriela
Master’s in advanced periodontics, Madrid European University, PhD student, Lecturer in the Clinical Dentistry Department of the Faculty of Biomedical Sciences, Madrid European University.