Update
Published in spanish Científica Dental Vol. 20. Nº 2. 2023
www.cientificadental.es
Antiplatelet therapy and oral surgery: to discontinue or not, that is the question
SUMMARY
Cardiovascular diseases are one of the most prevalent systemic disorders in the Western world. Many cardiopathy patients have had an acute coronary episode and are being treated with platelet antiaggregants. The therapy with these drugs can be a challenge for the odontologist, who must face an important dilemma: either maintain the drug with the consequent haemorrhagic risk, or withdraw it, with the possibility of thromboembolic complications, posing a risk to the patient’s life. Therefore, odontologists should know what should be the management of this type of patients before the prospect of performing a surgical procedure in the oral cavity or even a simple tooth extraction.
The objectives of this narrative review are, firstly, to recall the platelet physiology and the platelet thrombus formation mechanisms; secondly, to delve into the action mechanisms of the different antiplatelet drugs; and, thirdly, since there are no clinical guidelines on the subject, make a critical approach to the existing guidelines for a dental management of this type of patients, in order to prevent the occurrence of possible complications, not only local, but, more importantly, systemic complications. In these cases, before withdrawing antiplatelet therapy, it would be advisable to reduce the haemorrhagic risk versus the risk of generating a new thromboembolic episode, such as stent thrombosis or recurrence of the acute coronary accident, events that could put the patient’s life at risk.
Cardiovascular disease is the systemic disorder that generates greater morbidity and mortality in the western world. In Spain, according to data from the National Institute of Statistics, in 2020, cardiovascular disease was the leading cause of death among the general population, above tumours and COVID-19. In this sense, ischemic heart disease is the leading cause of death in men and stroke in women.
Among cardiovascular diseases, atherosclerotic pathology is the main cause of morbimortality in our country, including acute coronary syndromes, cerebrovascular diseases and peripheral arterial disease1. The etiopathogenic mechanism underlying all these pathologies is the rupture of the atheroma plaque, which triggers the platelet aggregation which is the cause of the acute thrombosis process. For this reason, in recent years, new antiplatelet drugs have been developed, which constitute the cornerstone of the recurrence prevention of acute ischemic episodes, both short-term and long-term. These drugs have to be known by the odontologist, especially when performing a surgical procedure, to know what should be the correct dental management to avoid potential complications, not only from the point of view of local bleeding, but also, most importantly, to avoid systemic complications (such as stent thrombosis or the appearance of a new thromboembolic event), which could put the patient’s life at risk2.
The purpose of this narrative review is, firstly, to recall the platelet physiology and the platelet thrombus formation mechanisms; secondly, to delve into the mechanisms of action of the different platelet antiaggregants; and, thirdly, since there are no clinical guidelines in this regard, make a critical approach to the existing guidelines for their correct dental management, in order to perform a surgical procedure in the oral cavity or even a simple tooth extraction, with sufficient guarantees of success.
Physiology of the platelet
The platelet is one of the blood forming elements, along with red and white blood cells. Normally, there are between 150,000 and 400,000 platelets per mcL in the blood, and the average platelet volume is usually 7-9 cubic micrometers. Platelets come from the hematopoietic stem cells of the bone marrow, specifically from the myeloid lineage and have an average life of 7 to 10 days. Inside they have alpha granules and dense granules, where molecules of special relevance accumulate in platelet physiology3.
Platelets play a primary role in haemostasis, since they initiate the repair of vascular lesions, forming the platelet plug, and also promote blood clotting, through the activation of thrombin released from the platelets themselves and the calcium released from the dense granules which are necessary for the formation of fibrin3.
Platelet physiology involves several enzymes, such as cyclooxygenase (COX), which transforms arachidonic acid (AA) from membrane phospholipids, in prostaglandins (PG), which are the thromboxane A2 (TXA2), vasoconstrictor and platelet proaggregant and prostacyclin (PGI2), which is a vasodilator and antiaggregant and originates in the vascular endothelium3. Other platelet enzymes include: phospholipase A2, which releases AA from membrane phospholipids and the phosphodiesterase, which hydrolyzes the cAMP.
The platelets have receptors in their membrane which are glycoproteins (GP) and are inactive under normal conditions. The most relevant are GP Ia and GP VI that bind to collagen, GPIb that binds to the von Willebrand factor (VWF) and GP IIb/IIla, which binds to several proteins, but the most important is fibrinogen. These receptors are involved in platelet adhesion phenomena (platelet binding to the injured vessel), activation (change of platelet morphology causing the secretion of granules) and aggregation (binding between several platelets)3.
Platelet thrombus formation mechanism
Platelets circulate in the bloodstream inactively. But when a vessel injury occurs, subendothelial collagen is exposed, which is the stimulus to recruit the platelets that will form the platelet plug. Remember that platelets do not adhere to the intact endothelium, but they can adhere to a foreign body inside the bloodstream (such as a coronary stent or a prosthetic heart valve)3.
Adhesion: When a vessel is injured, the circulating platelets slow down their speed over the damaged area, against the blood flow that pushes them, thanks to the platelet GPIb binding to the von Willebrand factor (VWF) of the matrix under the endothelium. Then, the subendothelial collagen establishes a more stable binding by binding to platelets3. GPIb and GPVI.
Activation: After adhesion, platelet activation occurs, appearing on the exterior of the receptors that were inactive. These activate intracellular molecules, which cause a change in platelet morphology, with the emission of pseudopods and release of certain substances that promote platelet aggregation, perpetuating the process. These molecules, known as platelet agonists are: TXA2, ADP and thrombin. Of all of them, ADP is the most potent for recruiting platelets and spreading arterial thrombus, for which it is considered a platelet activation amplifier. Platelets have on their surface three receptors for ADP: P2Y1, P2Y12 and P2X. Each induces different platelet signalling pathways, but P2Y12 is the most important since it favours the release of the content of the granules, the increase of intracellular calcium, the generation of TXA2 and the activation of the GPIIb-IIIa receptor, which is key in platelet aggregation. Consequently, the platelet P2Y12 receptor blockage is crucial to inhibit platelet activation and aggregation and thus prevent platelet thrombus formation. Therefore, in recent years new drugs have been developed capable of blocking this receptor1.
Release: After activation, the molecules stored in the granules of the platelets are released. Activated platelets can release up to 300 different proteins. From alpha granules, proteins homologous to plasma (fibrinogen, fibronectin, factor XIII, VWF) and platelet-specific proteins (platelet factor 4-FP4), thromboglobulin, P-selectin, PDGF (platelet-derived growth factor) and thrombospondin) are released. From the dense granules ADP, ATP, calcium and serotonin (5-hydroxytryptamine or 5-HT) is released3.
Aggregation: Once the platelets are trapped in the damaged area, new platelets are recruited from the bloodstream, known as platelet aggregation. Activation of the GPIIb/IIIa receptor is the final pathway leading to platelet aggregation. Once activated, it binds to its ligands, which have the sequence of RGD amino acids (Arg-Gly-Asp or arginine-glycine-aspartic), such as fibrinogen, but also VWF, fibronectin and vitronectin. This receptor is specific to platelets and binds in a bivalent manner to fibrinogen, forming binding bridges between two platelets3.
Regarding the mechanisms that regulate platelet aggregation, there are the contribution and inhibition factors.
Thus, they favour the platelet aggregation the ADP, the thrombin, the collagen, adrenaline and TXA2. While cAMP, cGMP and PGI2 inhibit it, as well as nitric oxide (NO), which in addition to being a platelet antiaggregant, is considered the most potent vasodilator of the organism3.
Platelet antiaggregants drugs (APAs)
Antiplatelet agents (APAs) are drugs that inhibit platelet aggregation, acting as antithrombotics. They usually act irreversibly and their function cannot be monitored, but it is usually considered that their effect lasts as long as the average life of the platelet, that is, between 7 and 10 days1. The main indications of these drugs include acute coronary syndromes (ACS) (which include acute myocardial infarction-AMI and unstable angina), stable coronary artery disease, cerebrovascular disease, and peripheral artery disease, but they are also used after surgical treatments that are performed after the appearance of these conditions, such as percutaneous coronary intervention (PCI) or revascularization surgery, and in the prevention of recurrence of the same, that is, in the secondary prophylaxis of atherosclerotic disease1.
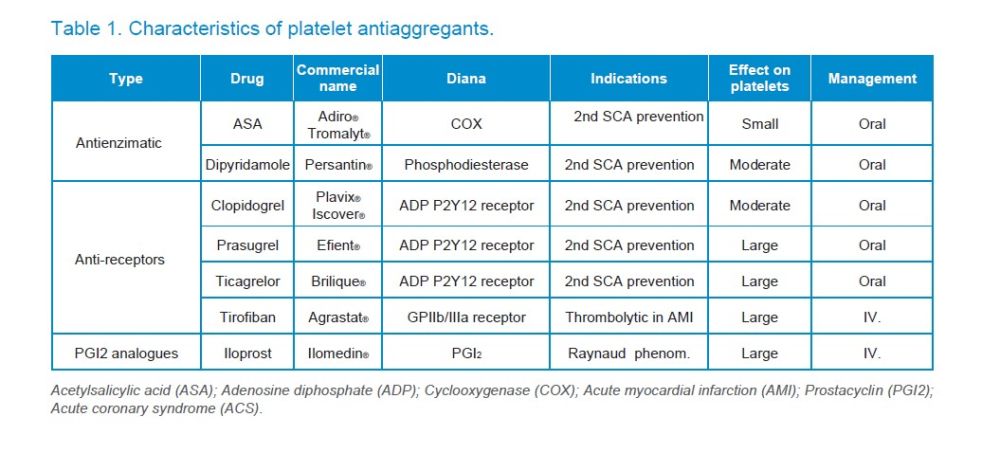
AAPs are drugs whose action mechanism is based on inhibiting platelet enzymes, such as COX (acetylsalicylic acid) or phosphodiesterase (dipyridamole), on the platelet P2Y12 receptor (such as thienopyridines) or GPIIb/IIIa receptor (such as tirofiban) or in acting as analogues of molecules that inhibit platelet aggregation (such as iloprost) (Table 1).
APAs can be classified according to their action mechanism in4-7:
1. Antienzimatic
1.1. Cyclooxygenase (COX) inhibitors
1.1.1. Acetylsalicylic acid (ASA) (Aspirin®, Adiro®, Tromalyt®)
1.1.2. Triflusal (Disgren®)
1.2. Phosphodiesterase inhibitors
1.2.1. Dipyridamole (Persantin®)
1.2.2. Cilostazol
1.2.3. Pentoxifylline
2. Receptor inhibitors
2.1. Of ADP (P2Y12): Ticlopidine, Clopidogrel, Prasugrel, Ticagrelor
2.2. From GPIIb/IIIa: Abciximab, Tirofiban, Eptifibatide
2.3. Of thrombin (PAR1): Vorapaxar
3. Prostacyclin analogues
3.1. Iloprost (Ilomedin®)
-
- Antienzimatic
1.1. COX inhibitors
1.1.1. Acetylsalicylic acid (Adiro® and Tromalyt®) Acetylsalicylic acid (ASA) is the antiplatelet par excellence. It is an irreversible inhibitor of the cyclooxygenase COX-1 and COX-2 of the platelet and, therefore, inhibits the synthesis of TXA2 platelet and of the PGI2 of the vascular endothelium, but especially of the first.
- Antienzimatic
In addition, small doses appear to affect only TXA2. Also, since platelets do not have a nucleus, they do not have the ability to resynthesize COX, unlike endothelial cells, for which the TXA2 inhibition lasts for the average platelet life, that is, between 7 and 10 days.
Inhibition of TXA2 only suppresses one of the aggregation mechanisms, but does not affect the aggregation induced by ADP. However, another effect of ASA in the
platelets is that it decreases the secretion of dense granules, that is, decreases the release of proaggregant substances during platelet activation. This would explain why its effects on platelets are more than one would expect from simple platelet inhibition dependent on a relatively weak agonist such as TXA21.
ASA is the basic antithrombotic therapy, used as an antiplatelet treatment only in the secondary prevention of atherosclerotic disease8.
1.1.2. Triflusal (Disgren®)
Triflusal is an ASA analogue, which selectively inhibits the platelet COX, but does not affect endothelial cells. Triflusal has fewer side effects than ASA, so it is indicated in patients with ASA resistance and in geriatric patients.
1.2. Phosphodiesterase inhibitors
1.2.1. Dipyridamole (Persantin®)
Dipyridamole is a phosphodiesterase inhibitor, which increases intracellular cAMP levels, inhibiting aggregation; it is also a vasodilator. It does not have advantages over ASA, but it can be associated with anticoagulant drugs and given to patients with cardiac valve prostheses with ASA intolerance.
1.2.2. Cilostazol
It increases intracellular cAMP levels and is a vasodilator.
1.2.3. Pentoxifylline
Pentoxifylline is a vasodilator phosphodiesterase inhibitor, currently used in the prevention of jaw osteonecrosis.
2. Platelet receptor inhibitors
2.1. Inhibitors of ADP P2Y12
2.1.1. Irreversible inhibitors: Thienopyridines
2.1.1.1. 1st Generation: Ticlopidine (Tiklid®) Ticlopidine is a thienopyridine derivative, which behaves like a prodrug, that is, it is metabolised in the liver resulting in an active
metabolite, which antagonizes ADP induced aggregation. It was the first inhibitor of the P2Y12 receptor, but the relative frequency of adverse reactions, such as diarrhoea and, above all, neutropenia (in 0.8% of cases), has made its use increasingly low.
2.1.1.2. Of 2nd generation: Clopidogrel (Plavix®, Iscover®)
It is a prodrug, which requires two oxidation reactions in the liver to transform into the active metabolite, which inhibits the P2Y12 receptor. However, a large individual variability has been described in the induced antiaggregation response by clopidogrel. It is usually used at a dose of 75 mg a day, being more powerful than 100 mg of ASA.
A coronary stent may be administered in conjunction with the ASA for the treatment of ACS after placing or after percutaneous revascularization surgery, which constitutes the so-called “dual antiplatelet therapy” or “dual platelet antiaggregation” (DPA).
2.1.1.3. Of 3rd generation: Prasugrel (Efient®)
Prasugrel is another prodrug that inhibits the P2Y12 receptor, and is more potent, faster and has less variability in the antiplatelet response than clopidogrel. It is the only one that has benefits in diabetics.
2.1.2. Reversible inhibitors
2.1.2.1. Ticagrelor (Brilique®)
Ticagrelor is an antagonist of the P2Y12 receptor with a reversible effect. It is faster and more powerful than clopidogrel. In addition, it has extraplatelet effects that are beneficial from a cardiovascular point of view.
2.1.2.2. Cangrelor
Recently, new antagonists even more potent antagonists like Cangrelor and Elinogrel have been designed. These new antiaggregants achieve greater antithrombotic efficacy, but also involve an increased risk of bleeding.
2.2. GPIIb/IIIa receptor inhibitor
2.2.1. Abciximab, Tirofiban, Eptifibatide
They are antiaggregants for hospital use administered by IV, blocking the binding of fibrinogen and VWF to the glycoproteins of the platelet surface (mediated by the GPIIb/IIIa receptor).
They are used as emergency thrombolytic drugs in the treatment of AMI. The sooner therapy with GPIIb/IIIa inhibitors is provided, the most favourable the prognosis for AMI.
2.3. PAR1 antagonist (protease 1 activating receptor)
2.3.1. Vorapaxar (Zontivity®)
It inhibits thrombin-mediated aggregation, since it is an antagonist of the thrombin PAR1 receptor. Accepted by the FDA, but not by the EMA.
3. Prostacyclin analogues
3.1. Iloprost (Ilomedin®)
Iloprost is an analogue of prostacyclin, which increases intraplatelet cAMP and is also a vasodilator. It is used in peripheral artery disease, thromboangiitis obliterans and Raynaud’s disease.
The Working Group on Cardiovascular Thrombosis of the Spanish Society of Cardiology8, coordinated by the Spanish Society of Cardiology (SEC) and made up of representatives of the majority of Spanish medical societies, considers that dental surgical procedures are of low haemorrhagic risk, since haemostasis can be achieved properly, a possible haemorrhage does not pose a vital risk to the patient or compromise the outcome of surgery and usually do not require transfusion. While some maxillofacial surgery procedures may be medium or high risk; medium risk would be when the haemorrhage may result in a transfusion or reoperation (such as removal of tumours, radical resection of the maxilla or jaw, or reduction of complicated bone fractures); and the high risk would exist in those surgeries in which the perioperative haemorrhage can be life-threatening for the patient or the outcome of surgery, such as in LeFort I, II or III surgery.
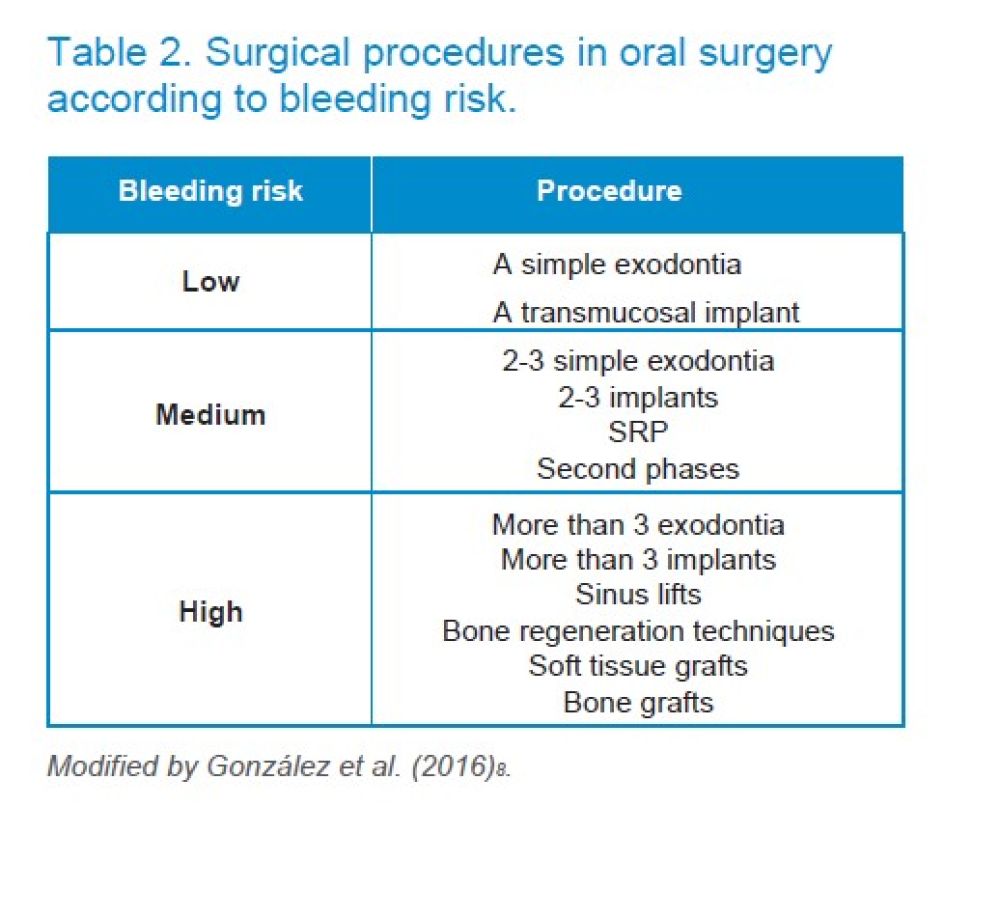
However, the authors of this study consider that the haemorrhagic risk of dental procedures should be stratified in another way. Therefore, we have classified the risk of bleeding following González et al9 in: low haemorrhagic risk, if a simple exodontia or transmucosal implant is to be done; medium risk, if less than 3 simple exodontia, less than 3 implants, second phases or scaling and root planning (SRP) are to be performed; and high risk if regenerative procedures are to be performed, soft and hard tissue grafts, sinus elevations, impacted teeth extractions, more than 3 implants or more than 3 extractions (Table 2).
The incidence of post-extraction bleeding rate in patients treated with AAP varies in literature from 0 to 17.4%10-13. However, it is necessary to take into account that in these patients the thrombotic risk is more important than the bleeding7 risk. Discontinuation of antiplatelet therapy may trigger thromboembolic events with serious consequences7. Stent thrombosis is a rare but potentially catastrophic event, leading to ACS or even death in 25-45% of the cases14, especially in the first 6 years of its placement8.
To stratify the thrombotic risk, the most important thing is the time elapsed since the transient ischemic attack, but the type of implanted stent must also be considered and how the ischaemic episode occurs. The lack of clinical trials should be noted8.
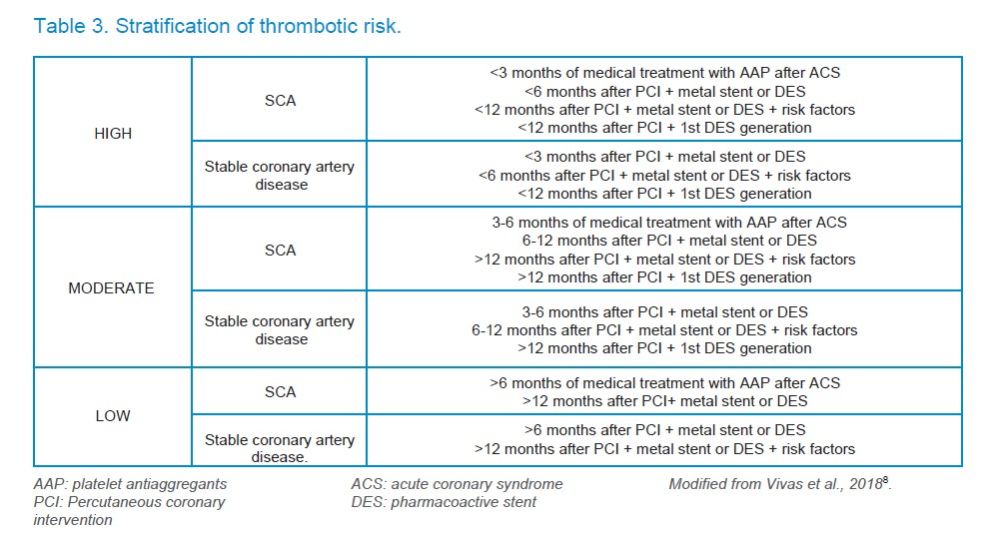
Patients with a stent are at higher risk of thromboembolic complications than those with a stable coronary artery disease7,14, especially when a drug-eluting stent or pharmacoactive stent (DES) has been inserted, especially the first generation, which are associated with a higher thrombosis possibility than the second generation, which have improved their safety profile8. According to Vivas et al.8, (2018), a high thrombotic risk has been reported when less than 3 months of ACS have passed and is only under medical treatment (especially during the first month) or less than 6 months from the implantation of a second generation DES or less than 12 months from the first generation DES. The thrombotic risk will be moderate when 3 to 6 months have passed after the ACS under medical treatment, between 6 and 12 months after placement of second-generation ACS or more than one year of first-generation ACS. Thrombotic risk is considered low if more than 6 months of ACS under medical treatment or more than one year since the placement of second-generation ACS8 (Table 3) have passed.
In general, without having to consult the cardiologist for the type of stent, the thrombotic risk can be summarized as: high if less than 6 months have passed since the implantation of any type of stent; medium risk, if 6 to 12 months have passed since the implantation of any type of stent; and low risk, if more than 12 months have passed since the implantation of any stent or if it does not have a stent.
In general, the carriers of one or more stents undergo double antiplatelet therapy (DAPT) during the year following the implantation surgery8, for which during this period their thrombotic risk is considered moderate to high and also their bleeding risk.
The removal of ASA produces a rebound effect on the platelet physiology, in such a way that it decreases fibrinolysis and increases TXA26 production. Each day that the ASA or clopidogrel is suspended, a platelet regeneration of 15-20%3 occurs, that is, after stopping the antiplatelet therapy, platelet aggregation returns to baseline level after 5 days6.
ASA
Haemorrhagic risk
Omar et al.15, in 2015, observed that taking ASA did not increase the risk of bleeding in patients who underwent the extraction of all the teeth (full-mouth extraction), recommending to continue taking ASA and use local haemostatic measures.
In the study of Lu et al.16 (2015), the incidence of haemorrhage in the group with ASA was 1.2% vs 0.7% in the control group, so they do not advise stopping the drug.
Eapen et al.17, in 2017, designed a prospective study with 80 patients receiving a low ASA dose treatment for tooth extractions. In one group the ASA was stopped and the other continued the treatment. In no case was there prolonged postoperative bleeding and only one case with ASA local haemostatic measures had to be performed. This group recommends not to interrupt ASA before tooth extraction.
In the prospective study by Gupta et al.18, 2018, they concluded that stopping ASA prior to tooth extractions is not necessary, as local haemorrhage can be solved with local haemostatic measures.
Thrombotic risk and ASA interruption
In the meta-analysis by Burger et al.19, 2.3% to 6.1% of acute cardiovascular events were observed when ASA was stopped before surgery.
In the meta-analysis by Biondi-Zoccai et al.20, with more than 50,000 patients it was found that the interruption of ASA produced adverse cardiovascular effects, so they advised not to stop ASA therapy.
According to Mahmood et al.7, ASA should not be stopped, especially if it is indicated in secondary prevention of ACS, stroke or after revascularization surgery7.
For patients with simple antiaggregation (SAA), it is recommended to continue with ASA, since it has been shown to reduce the ischemic risk without significantly increasing the risk of bleeding, according to the Working Group on Cardiovascular Thrombosis of the Spanish Society of Cardiology8.
Clopidogrel
Haemorrhagic risk
Omar et al.15 observed that Clopidogrel administration did not increase the risk of bleeding in patients who had all their teeth extracted (full-mouth extraction), recommending to maintain Clopidogrel and use local haemostatic measures.
Thrombotic risk and Clopidogrel interruption
Stent carriers have a higher risk of thrombotic complications, especially with drug coatings. There are examples in the literature where it is reported that after stopping Clopidogrel, a stent thrombosis occurs14,21,22.
Clopidogrel discontinuation is a risk factor for stent thrombosis. When the patient is in AAP treatment only with Clopidogrel it is advised not to stop it7,
When the thrombotic risk is high or moderate, Clopidogrel should not be stopped before dentoalveolar surgery7. It is preferable to postpone surgery until the thrombotic risk is low8. When more than 12 months have passed since stent insertion and the thrombotic risk is low, if the dental procedure involves a low haemorrhagic risk, such as a simple exodontia, clopidogrel should not be stopped. But if the surgical procedure is regenerative surgery with a high bleeding risk, the cardiologist should be consulted to discontinue Clopidogrel 5 days before surgery and take it again after 24-48 hours.
DAP
Haemorrhagic risk
Lillis et al.23 compared bleeding after tooth extractions in patients with DAP vs SAA and observed increased bleeding in DAP. However, all cases were successfully handled with local measures.
In the study by Lu et al.16, the incidence of bleeding in the DAP group was 4.4% vs 0.7% in the control group, however, they do not advise discontinuing this treatment before tooth extractions.
Napeñas et al.24, found no significant differences in intraoperative bleeding between the DAP and SAA groups, although they observed greater bleeding in the immediate postoperative period in the DAP group. His opinion is that it is not necessary to stop dual antiplatelet therapy before the dental surgical procedure.
Olmos-Carrasco in 201810 found 8.3% of haemorrhagic complications in the first 30 minutes after tooth extraction in patients with DAP, which were resolved with local haemostatic measures.
Nathwani and Martin in 201625 conducted a literature review and in the consulted articles all cases of bleeding under DAP were resolved with local measures, so they do not advise to interrupt the antiaggregant.
In the Ockerman systematic review of 201926, they found greater postoperative bleeding with DAP than with SAA, but all cases were solved with local measures and the authors do not recommend stopping any DPA before exodontia.
Sánchez-Palomino et al.12 consider that an impregnated gauze in tranexamic acid (Amchafibrin®) for 30 minutes is advisable to avoid postoperative bleeding in patients with SAA and DAP.
Mahmood et al. in 20207 commented that there is no article of uncontrolled bleeding after dental surgery in patients under DAP and conclude that there is no indication to stop DAP prior to a surgical procedure of the oral cavity.
Thrombotic risk and DAP discontinuance
In the article jointly published by the American Dental Association, the American Heart Association, the American College of Cardiology, the Society for Cardiovascular Angiography & Interventions and the American College of Surgeons27 highlighted the importance to continue with DAP in patients with coronary stents. Discontinuation of dual treatment with aspirin and clopidogrel in patients with stents is associated with a 5 to 10 higher risk of myocardial infarction and even mortality. This risk is inversely proportional to the time elapsed since stent insertion. The thrombosis risk is greater than the risk of bleeding, so stopping ASA or clopidogrel should be avoided27.
In patients under DAP, dental surgeries should be postponed until they stop this therapy. They can only undergo surgery if their life depends on the same and without interrupting the DAP7. In the systematic review by Childers et al.28, they reaffirm the need to conduct a risk-benefit assessment before performing dental surgery with these patients.
According to the Working Group on Cardiovascular Thrombosis of the Spanish Society of Cardiology8, which seeks a consensus to homogenize protocols, the first consideration with patients under PAD is to assess the need for elective intervention while the thrombotic risk is moderate to high; if the intervention can be delayed, it is best to postpone it until the thrombotic risk of the patient is considered low.
When the thrombotic risk is low, that is, more than 12 months have passed after the implantation of any stent, but the cardiologist has preferred to maintain the DAP beyond the first year, and they will undergo minor dental surgical procedures, with low bleeding risk, as a simple extraction, ASA and clopidogrel should be maintained. If the thrombotic risk is low and the bleeding risk is moderate to high, for example, in a regenerative surgery procedure, the possible discontinuance of clopidogrel 5 days before surgery will have to be discussed with the cardiologist.
For many years, dentists have overestimated the risk of local bleeding when performing exodontia or implants in patients who are taking antiaggregants and, on the other hand, we have underestimated the risk of thrombosis, promoting the discontinuance of the drug between 5 and 7 days before the surgical procedure. However, the current recommendations go in the opposite direction, since it has been seen that when antiaggregants are stopped, new cardiovascular events can occur2,6,7,27.
Regarding thrombotic risk, surgical procedures may be performed only when it is low, that is, when more than one year has passed after the stent placement8. if not more than one year has passed, it is preferable to postpone dental surgery6,8.
It should be borne in mind that, at present, the single antiaggregant medication should not be stopped before performing an exodontia or a surgical procedure in the oral cavity and, if modified, it should be as little as possible and after consultation with the cardiologist17,18,23.
When a patient is being treated with ASA at low doses (100-300 mg/ day), it is not stopped6,7,19.
When a patient is exclusively taking clopidogrel (75 mg) and dental extractions or surgical procedure are required, it is not stopped. Only if the thrombotic risk is low and regenerative surgery procedures are required can the drug be satopped for 5 days after consultation with the cardiologist8,15.
If the patient is receiving dual antiplatelet therapy with ASA plus clopidogrel, it is because they have had one or more stents inserted in the last year, therefore, it will have a moderate to high thrombotic risk and it is not advisable to discontinue any of the drugs prior to an exodontia or surgical procedure of any kind26-30. It is preferable to postpone the surgical procedure6,8.
If the patient has a low thrombotic risk and a low bleeding risk is expected, SAA should be continued with both ASA and clopidogrel. If the patient is still under DAP after more than one year of the stent insertion, the cardiologist who knows the hemodynamic state and the type of stent the patient has, should be consulted to be able to discontinue exclusively the clopidogrel 5 days before the dental surgical procedure. The ASA will always be continued.
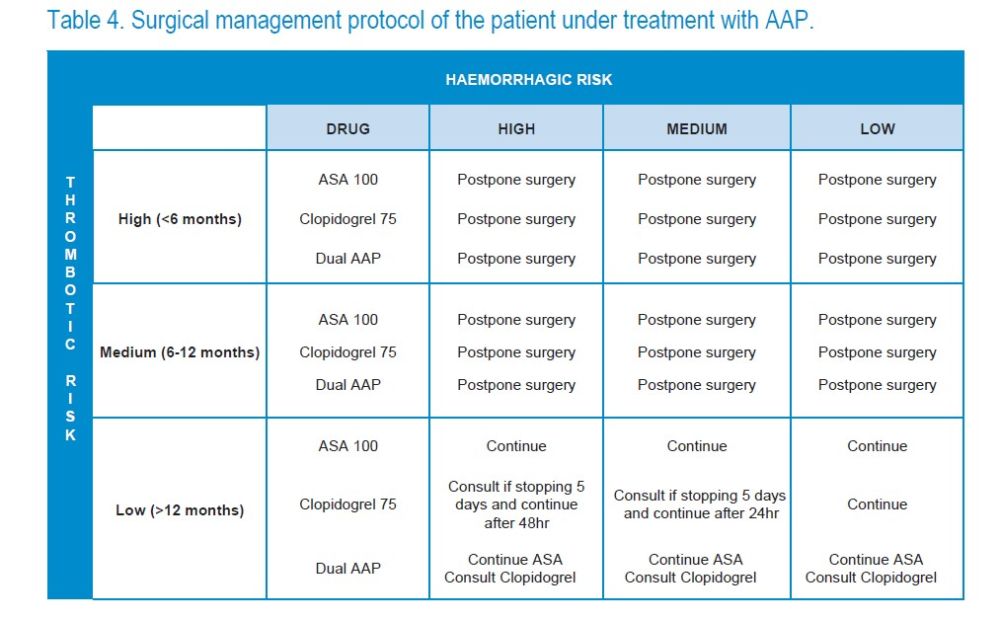
Each case should be individualized, but in general6–15-4 (Table 13):
1. When the patient is treated with ASA 100- 300 mg it is always continued.
2. When the patient is taking clopidogrel at low doses (75 mg/ day), it is not stopped.
3. If the patient is being treated with another AAP more potent than ASA or clopidogrel (ticagrelor or prasugrel), the cardiologist should be consulted.
4. When the thrombotic risk is high (for example, a patient with a stent that has been inserted for less than 6 months) and the patient is undergoing DAP, none of the AAPs can be stopped. It is preferable to postpone the procedure8.
5. If the thrombotic risk is moderate (for example, a patient who has a stent that has been inserted between 6 and 12 months), according to the Spanish Society of Cardiology, the ideal is to wait for the thrombotic risk to be low8.
6. If the thrombotic risk is low and the bleeding risk is expected to be low, such as exodontia, antiaggregants are maintained.
7. If the thrombotic risk is low, but the haemorrhagic risk is expected to be moderate to high, as in regenerative procedures, the cardiologist should be consulted to stop clopidogrel 5 days before the procedure and resume it after 48 hours, if the bleeding risk is high after 24 hours, or if the risk is moderate8.
8.When the patient is undergoing a triple antiaggregant therapy, the cardiologist should be consulted.
9.In all cases it will be necessary to carry out local haemostatic procedures that, according to the consulted authors, are effective to prevent local haemorrhagic complications6-19,24-31.
10.Local tranexamic acid is an effective option to reduce the bleeding risk in patients who are being treated with platelet antiaggregants12.
Badimon L, Vilahur G. Mecanismos de acción de los diferentes agentes antiplaquetarios (Action mechanisms of different antiplatelet agents). Rev Esp Cardiol 2013;13(B):8-15.
Ferrari E, Benhamou M, Cerboni P, Marcel B. Coronary syndromes following aspirin withdrawal: a special risk for late stent thrombosis. J Am Coll Cardiol 2005; 45: 456- 9.
López Farré A, Macaya C. Plaqueta: Fisiología de la activación y la inhibición. Rev Esp Cardiol 2013;13(B):2-7.
Jourdi G, Godier A, Lordkipanidze M, Marquis-Gravel G, Gaussem P. Antiplatelet Therapy for Atherothrombotic Disease in 2022. From Population to Patient-Centered Approaches. Front Cardiovasc Med. 2022 https://doi. org/10.3389/fcvm.2022.805525.
Badimon L, Mendieta G, Vilahur G. Diferencias en los mecanismos de acción de los nuevos antiagregantes: ¿cómo actúan? Rev Esp Cardiol Supl 2014;14 (A):3-9.
Ganthous AE, Ferneini EM. Aspirin, Plavix, and Other Antiplatelet Medications. What the Oral and Maxillofacial Surgeon Needs to Know. Oral Maxillofacial Surg Clin N Am. 2016 http://dx.doi.org/10.1016/j. coms.2016.06.003
Mahmood H, Siddique I, McKechnie A. Antiplatelet drugs: A review of pharmacology and the perioperative management of patients in oral and maxillofacial surgery. Ann R Coll Surg Engl 2020; 102(1): 9–13.
Vivas D, Roldán I, Ferrandis R, et al. Manejo perioperatorio y periprocedimiento del tratamiento antitrombótico: documento de consenso de SEC, SEDAR, SEACV, SECTCV, AEC, SECPRE, SEPD, SEGO, SEHH, SETH, SEMERGEN, SEMFYC, SEMG, SEMICYUC, SEMI, SEMES, SEPAR, SENEC, SEO, SEPA, SERVEI, SECOT
y AEU. Rev Esp Cardiol. 2018;71(7):553- 564.
González Fernández-Tresguerres F, Alvarez Sirvent A, Torres J, Fernández- Tresguerres I. Nuevos anticoagulantes orales: repercusión odontológica. Científica Dental 2016;13:35-43.
Olmos-Carrasco O, Pastor-Ramos V, Espinilla-Branco R, et al. Hemorrhagic complications of dental extractions in 181 patients undergoing double antiplatelet therapy. J Oral Maxillofac Surg 2015;73:203-10.
Cardona-Tortajada F, Sainz-Gomez E, Figuerido-Garmendia J, et al. Dental extractions in patients on antiplatelet therapy. A study conducted by the Oral Health Department of the Navarre Health Service (Spain). Med Oral Patol Oral Cir Bucal 2009;14:e588.
Sanchez-Palomino P, Sanchez-Cobo P, Rodrigues-Archilla A, et al. Dental extraction in patients receiving dual antiplatelet therapy. Med Oral Patol Oral Cir Bucal 2015;20:e616.
Yanamoto S, Hasegawa T, Rokutanda S, et al. Multicenter Retrospective Study of the Risk Factors of Hemorrhage After Tooth Extraction in Patients Receiving Antiplatelet Therapy. J Oral Maxillofac Surg 2017;75:1338-43.
Iakovou I, Schmidt T, Bonizzoni E et al. Incidence, predictors, and outcome of thrombosis after successful implantation of drug-eluting stents. JAMA 2005;293(2):126–130.
Omar HR, Socias SM, Powless, RA, Sprenker C, Karlnoski R, Mangar D, Camporesi EM. Clopidogrel is not associated with increased bleeding complications after full-mouth extraction: A retrospective study. J Am Dent Assoc. 2015; 146:303-9.
Lu SY, Tsai CY, Lin LH, Lu SN. Dental extraction without stopping single or dual antiplatelet therapy: Results of a retrospective cohort study. Int J Oral Maxillofac Surg. 2016;45:1293-8.
Eapen BV, Baigi MF, Avinash S. An Assessment of the Incidence of Prolonged Postoperative Bleeding After Dental Extraction Among Patients on Uninterrupted Low Dose Aspirin Therapy and to Evaluate the Need to Stop Such Medication Prior to Dental Extractions. J Maxillofac Oral Surg. 2017;16(1):48-52.
Gupta R, Dugal A, Sane VD, Hiwarkar S, Khandelwal S, Iyengar A. Effect of Low-Dose Aspirin on Bleeding Following Exodontia: A Prospective Clinical Study. J Maxillofac Oral Surg. 2018;17(3):350- 355.
Burger W, Chemnitius M, Kneissl GD, Rucker G. Low-dose aspirin for secondary cardiovascular prevention- cardiovascular risks after its perioperative withdrawal versus bleeding risks with its continuation- review and meta-analysis. J Int Med. 2005;257:399-414.
Biondi-Zoccai GG, Lotrionte M, Agostoni P, et al. A systematic review and meta-analysis on the hazards of discontinuing or not adhering to aspirin among 50,279 patients at risk for coronary artery disease. Eur Heart J 2006;27(22):2667–74.
Pennacchi M, Stio RE, Lucisano L, Calcagno S, Mancone M, Sardella G. Five years of dual antiplatelet therapy DES thrombosis after clopidogrel withdrawal. Int Heart J 2013;54:234-6.
Artang R, Dieter RS. Analysis of 36 reported cases of late thrombosis in drug-eluting stents placed in coronary arteries. Am J Cardiol 2007; 99(8):1039–43.
Lillis T, Ziakas A, Koskinas K, Tsirlis A, Giannoglou G. Safety of dental extractions during uninterrupted single or dual antiplatelet treatment. Am J Cardiol 2011; 108:964-7.
Napeñas JJ, Oost FC, DeGroot A, et al. Review of postoperative bleeding risk in dental patients on antiplatelet therapy. Oral Surg Oral Med Oral Pathol Oral Radiol 2013; 115:491–9.
Nathwani S, Martin K. Exodontia in dual antiplatelet therapy: the evidence. Br Dent J 2016;220(5):235-8.
Ockerman A, Bornstein MM, Leung YY, Li SKY, Politis C, Jacobs R. Incidence of bleeding after minor oral surgery in patients on dual antiplatelet therapy: a systematic review and meta- analysis. Int J Oral Maxillofac Surg 2020;49(1):90-8.
Grines CL, Bonow RO, Casey DE Jr., et al. Prevention of premature discontinuation of dual antiplatelet therapy in patients with coronary artery stents: a science advisory from the American Heart Association, American College of Cardiology, Society for Cardiovascular Angiography and Interventions, American College of Surgeons, and American Dental Association, with representation from the American College of Physicians. J Am Dent Assoc 2007;138(5):652-5.
Childers CP, Maggard-Gibbons M, Ulloa JG et al. Perioperative management of antiplatelet therapy in patients undergoing non-cardiac surgery following coronary stent placement: a systematic review. Syst Rev 2018;7:4.
Park M, Her S, Kwon J. Safety of dental extractions in coronary drug-eluting stenting patients without stopping multiple antiplatelet agents. Clin Cardiol 2012; 35:225-230.
Sáez-Alcaide LM, Sola C, Molinero- Mourelle P, Paredes-Rodríguez V, Zarrias-Caballero C, Hernández- Vallejo G. Dental management in patients with antiplatelet therapy: A systematic review. J Clin Exp Dent 2017;9(8):e1044-e1050.
Wahl MJ. Dental surgery and antiplatelet agents: bleed or die. Am J Med 2014; 127:260-7.

González Fernández- Tresguerres, Francisco
Master in Oral Medicine Universidad Complutense de Madrid (UCM). Master’s
Degree in Oral Surgery and Implantology UCM. International Doctor Mention UCM. Honorary collaborator of the Department of Clinical Dental Specialties. Faculty of Dentistry, UCM.
Serrano Zamora, Rebeca
Master’s Degree student in Oral Surgery and Implantology UCM and PhD student, UCM.
Baca González, Laura
Master’s Degree in Oral Implantology UCM. Master’s Degree in Oral Surgery and Implantology UCM, PhD student UCM.
Iglesias Velázquez, Óscar
Master’s Degree in Oral Medicine
UCM. Master’s Degree student
in Oral Surgery and Implantology
UCM. Assistant in the Department
of Clinical Dental Specialities.
Faculty of Dentistry, UCM.
Xing Gao, Baoluo
Master’s Degree in Oral Surgery and Implantology UCM, PhD student UCM.
Fernández-Tresguerres Hernández-Gil, Isabel
Associate Professor and Doctor in the Department of Clinical Dental Specialities. Faculty of Dentistry, UCM.