Case report
Published in spanish Científi ca Dental Vol. 21. Nº 1. 2024
www.cientificadental.es
Clinical, radiographic and histomorphometric behaviour of the autologous tooth as a biomaterial in lateral access maxillary sinus elevation. Case report with six months of post-prosthetic loading follow-up
Introduction: Bone loss after extractions may require a sinus elevation to be performed in the posterior maxilla for the correct placement of implants. Autologous bone is considered the gold standard, but has a high rate of resorption and morbidity, leading to other alternatives such as autologous tooth, with good results in regenerative procedures. This case report evaluates at the clinical, radiographic and histomorphometric level the use of the autologous tooth in maxillary sinus elevation and the behaviour of two implants placed in a delayed manner.
Case report: The case is presented of a 48-year-old woman who came for consultation to replace the right posterior sector. The extraction of 4.8 as a donor tooth was performed to use it as a biomaterial in a lateral access sinus elevation, placing two implants six months after the intervention, and evaluating them six months after their prosthetic loading.
Discussion: The autologous tooth in this case report showed 30.56% of newly formed bone following a six-month wait, with better results than when allografts and xenografts were used. In addition, different cultural and ethnic aspects support the acceptance of the autologous tooth by patients. However, more long- term studies are needed to evaluate the stability of this type of graft in maxillary sinus elevation.
Conclusions: The autologous tooth in the sinus elevation offers biocompatibility, low incidence of complications and good patient acceptance, with good clinical and radiographic behaviour of the implants, despite the short time elapsed in this case after loading.
Three months after dental extraction, there is a loss of 50% of the initial bone dimensions of the socket, which is particularly significant in the posterior region of the maxilla. In this anatomical region, the loss of antral teeth results in three-dimensional pneumatization of the maxillary sinus, which may extend to the alveolar crest and the anterior region, the tuberosity area, and the zygomatic bone. This dual process of pneumatization and bone remodelling reduces bone availability both horizontally and vertically, potentially compromising implant treatment and its long-term stability1-6.
In such cases, the most predictable technique for bone reconstruction is the maxillary sinus elevation, which enables correct placement of implants and subsequent implant-supported restoration, thereby improving the quantity and quality of bone at the implant site. Among the maxillary sinus elevation techniques, the lateral approach is indicated when the vertical bone height is ≤ 4 mm, with delayed placement of the implants, whereas with a height
≥ 5 mm, the transcrestal sinus elevation technique and shorter implants are recommended, or the open technique with simultaneous placement of longer implants7,8.
The lateral approach sinus lift, also known as the open technique, is a well-documented procedure, having been described by Tatum9 in 1976 and subsequently published by Boyne and James10 in 1980. This consists of raising a full-thickness flap to access the anterolateral wall of the maxillary sinus, and, by means of osteotomy, creating a window in the buccal cortical bone to expose the Schneiderian membrane. Once this membrane is exposed, it is carefully detached and elevated until it reaches a horizontal position to form the new sinus floor, after which a graft biomaterial is placed. A membrane may be placed, either resorbable or non-resorbable, prior to suturing, to prevent displacement of the graft and colonisation of the sinus interior by periosteum originating from the flap11,12.
Among the biomaterials employed in this technique, autologous bone is currently regarded as the gold standard, as it provides an effective scaffold for osteoconduction, contains growth factors to promote osteoinduction, and osteocompetent cells to facilitate osteogenesis. However, certain disadvantages, such as donor site morbidity, limited availability, and a high rate of resorption, may restrict its use13,14.
For these reasons, various bone substitutes have been utilised (allografts, xenografts, and alloplastic materials). Most of these biomaterials exhibit only osteoconductive properties and have highly variable resorption times, ranging from very short (derived from polyglycolic and polylactic acid) to very long (hydroxyapatites), whilst others may provoke immune reactions (allografts). Owing to these disadvantages, studies on the clinical behaviour of tooth material in various regenerative procedures have increased in recent years, due to its similarity to human bone15,16.
Kim et al.17 described the osteoinductive and osteoconductive properties of tooth material, as well as lower morbidity and greater patient acceptance, with its favourable clinical and radiographic behaviour having been demonstrated in maxillary sinus elevation procedures, guided bone regeneration, and alveolar preservation18.
The objective of this clinical case is to evaluate, clinically, radiographically, and histomorphometrically, the use of autologous tooth as a biomaterial in maxillary sinus elevation, as well as the clinical and radiographic behaviour of two implants placed in a delayed manner following the sinus elevation, and their progress six months after prosthetic loading.
This case report presents a 48-year-old woman who attended the clinic for restoration of the posterior region of the first quadrant.
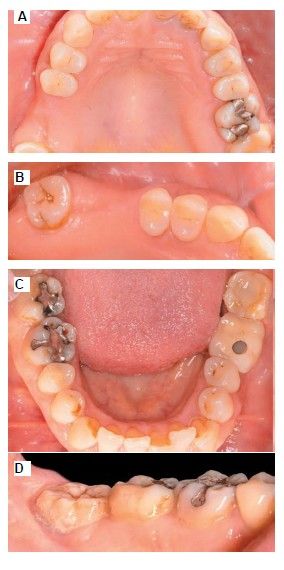
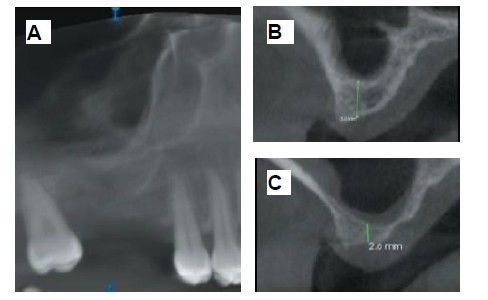
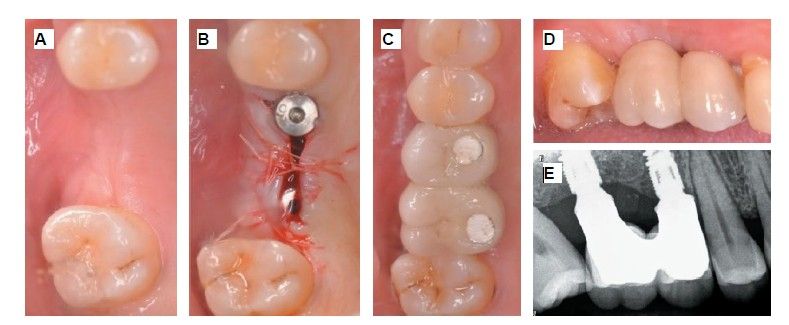
The medical history revealed no relevant medical or surgical antecedents, no known drug allergies, and no harmful habits. Intraoral examination revealed the absence of 1.6 and 1.7, and the presence of 1.8 and 4.8 (Figure 1). Radiographic examination using cone beam computed tomography (CBCT) revealed a residual height of 5.0 mm at 1.6, where an implant could be placed simultaneously, and 2.6 mm in the region of 1.7, making simultaneous implant placement with the lateral approach sinus elevation difficult (Figure 2).
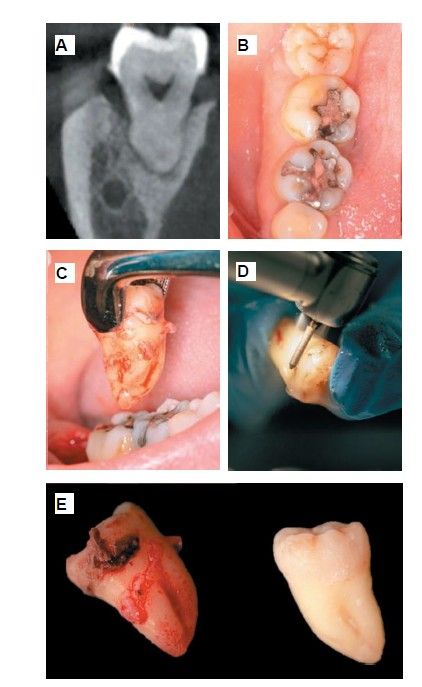
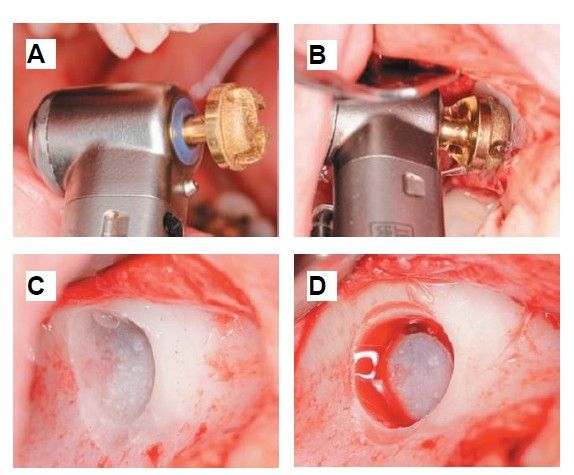
Extraction of 4.8 was planned in order to use it as the donor tooth, for which informed consent was obtained in advance. An anaesthetic block was administered using 4% articaine (Inibsa®, Barcelona, Spain) with 1:100,000 adrenaline to the inferior dental nerve, the lingual nerve, and finally the buccal nerve. As the tooth had no associated infectious processes, only calculus was removed from the tooth using ultrasonic instrumentation and the extraction was performed as atraumatically as possible. The root surface was polished with turbine diamond burs under copious irrigation, thereby removing the periodontal ligament (Figure 3).
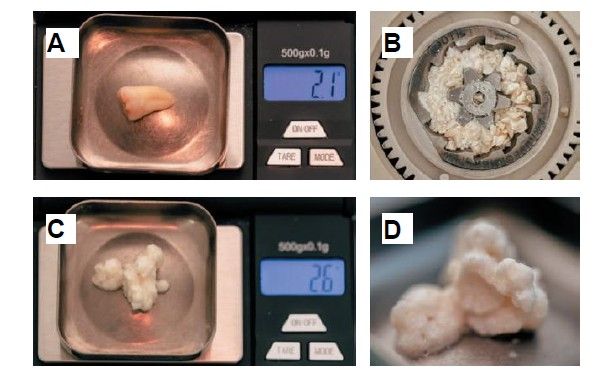
The weight of the tooth, once cleaned, was recorded using a precision balance (Ohaus® YA 102, YA Gold Series, New Jersey, USA), registering a weight of 2.1g. The tooth was then sectioned into fragments ≤ 5 mm, which were placed dry into the mill of the Tooth Transformer® device (S.R.L., Milan, Italy), as indicated by the manufacturer. Once introduced, it was placed inside the device and the container with the liquids was added, in order to demineralise the tooth, releasing BMP-2 and type I collagen, and eliminating any residual toxicity. When all components were inserted, the machine cover was closed and, by pressing the activation button, the process was initiated until the grinding of the fragments and the appropriate particle size was confirmed, thanks to the sieve present in the collecting container (400–800μm). Within 25 minutes, the autologous tooth graft was prepared and reweighed on the precision balance, recording a weight of 2.6g (Figure 4).
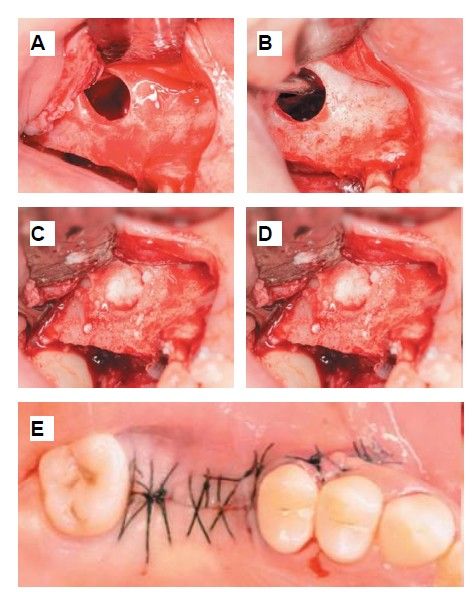
During the preparation of the tooth in the Tooth Transformer® device, a lateral approach sinus elevation was performed using an anaesthetic block with 4% articaine and 1:100,000 adrenaline (Inibsa®, Barcelona, Spain) of the posterior and middle superior alveolar nerves and the greater palatine nerve. Following a partial Neumann incision with a vertical release at the mesial angle of tooth 1.5, a mucoperiosteal flap was raised, and a controlled osteotomy was performed using the Sinus Master III® system (MCTBIO, Gyeonggi-do, 17037, South Korea), employing hydraulic pressure and diamond burs. A processed tooth graft was placed inside the maxillary sinus, and a Lyoplant® resorbable collagen membrane (B. Braun Medical S.A., Barcelona, Spain) was positioned over the graft. After this step, suturing was performed using 4/0 non-resorbable monofilament suture (Supramid®, B. Braun, Barcelona, Spain) (Figures 5 and 6).
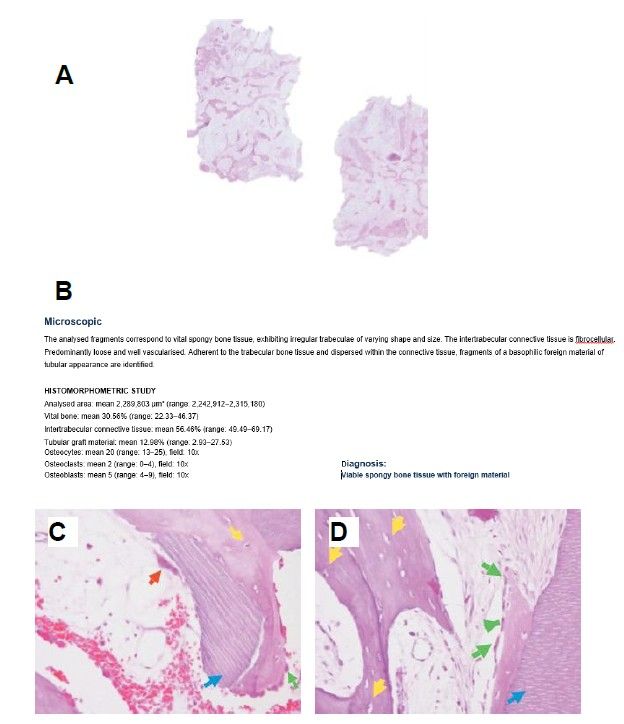
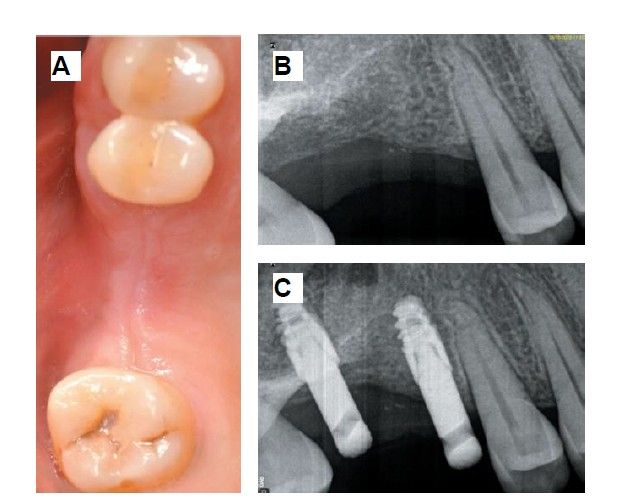
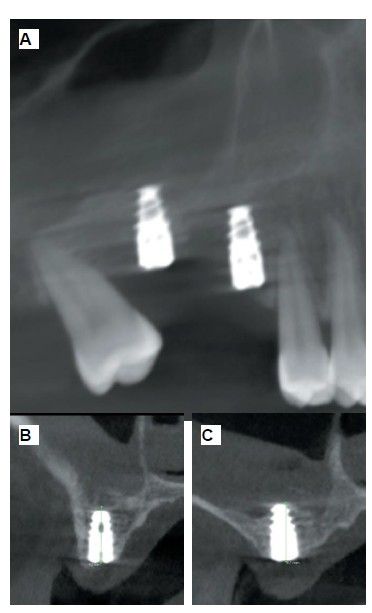
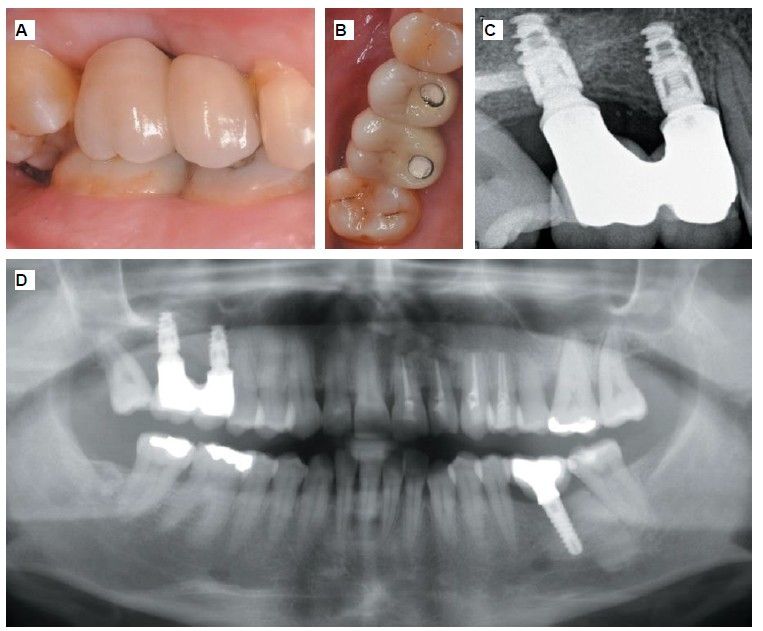
Six months after the maxillary sinus elevation surgery, re-entry was performed for the placement of implants. A 3 x 7mm bone tissue biopsy was obtained using a trephine, and histomorphometric analysis was requested, revealing 30.56% vital bone (Figure 7). After the biopsy sample was taken, two bone level Naturactis implants from ETK® (ETK Implants S.L, Sant Boi de Llobregat, Spain) were placed with an insertion torque of 35 Ncm, and their correct positioning was confirmed in the immediate postoperative period by means of a periapical radiograph (Figure 8). Six months after the placement of the implants, the patient was able to attend for the second stage to place healing abutments. A verification CBCT was performed to assess the final bone height, revealing an increase of 4.2 mm in the region of 1.6 and 6.1 mm in the region of 1.7 (Figure 9).
Fifteen days after the second stage, impressions were taken for the fabrication of two splinted cement-screw-retained crowns on titanium bases, with the fit verified by a parallelised periapical radiograph (Figure 10). Six months after placement of the restoration, a clinical and radiographic review was conducted, noting the favourable condition of the soft tissues (Figure 11).
In recent decades, the use of biomaterials derived from dental structures, such as dentine and enamel, has been investigated in various bone regeneration procedures. This approach is based on the autologous nature of this material, eliminating the need for a second donor site, and on the structural and chemical similarity between tooth and bone tissue, which confers osteoconductive and osteoinductive properties.
Chemically, the inorganic composition of dentine is 70% compared to 65% in autologous bone, while the organic component of dentine is 20% compared to 25% in autologous bone, with a water content of 10% common to both. The inorganic content is primarily hydroxyapatite, while the organic matter consists mainly of type I collagen, as well as bone morphogenetic proteins (BMP)19.
Some authors consider that different demineralisation processes favour the release of insulin-like growth factors (IGF), bone morphogenetic protein type 2 (BMP-2), transforming growth factor beta (TGF-beta), and type I collagen, all of which are directly involved in osteoinduction and angiogenesis. The mechanisms by which demineralised dentine stimulates bone regeneration are quite similar to the formation of newly formed bone when using autologous bone. Following demineralisation, both the bone matrix and the demineralised dentine matrix have an increased capacity to release type I collagen, growth factors, and BMP-2, thereby providing osteoinduction in regenerative procedures20,21.
The inorganic content, consisting of four types of calcium phosphates (amorphous calcium phosphate, hydroxyapatite, octacalcium phosphate, and tricalcium phosphate), imparts osteoconductivity to the tooth, permitting a low resorption rate, lower than that of autologous bone, thereby ensuring greater stability over time20,22.
Although the use of autologous bone in bone regeneration continues to be regarded as the gold standard among biomaterials, the use of tooth, compared to other biomaterials employed in maxillary sinus lift procedures, such as xenografts, has resulted in greater bone formation and a lower amount of residual biomaterial. Moreover, tooth as a biomaterial demonstrates a greater quantity of osteoid tissue surrounding the particles of the treated tooth, which are subsequently replaced by a greater amount of newly formed bone over time18,23-26.
The use of autologous bone in lateral approach sinus lift has demonstrated a higher rate of resorption compared to other biomaterials. Pesce et al.27, in their 2021 systematic review, confirmed the varying rates of volumetric reduction among different biomaterials after a six-month waiting period, with xenograft being the material that exhibited the least volume reduction (7.30 ± 15.49%) and autologous bone experiencing the greatest volumetric reduction (41.71 ± 12.63%), with alloplastic grafts (27.82 ± 15.58%) and allografts (30.23 ± 1.61%) falling in between. Indeed, due to the high rate of resorption associated with autologous bone, Khijmatgar et al.28, observed improved performance and a lower resorption rate when it was combined with different biomaterials (xenograft, alloplastic materials).
The percentage of newly formed bone obtained using the autologous tooth as a biomaterial in the present clinical case is 30.56%, six months after the lateral approach maxillary sinus lift. This value is similar to that reported by other authors employing the tooth as a biomaterial, such as Jun SH et al.29, with 31.07 ± 14.52% after four months post-intervention, or Minetti et al.30with 36.28%
± 9.77% after six months. Conversely, the amount of newly formed bone when using alloplastic grafts is 20.3–33.40% after six months of healing, 22.8% when using equine-derived xenograft, and 16.1–23.02% in the case of bovine-derived xenografts. However, the use of allografts yields higher percentages of newly formed bone, around 20.47–32.1%31-24.
In a recent in vitro study, the physicochemical and biochemical characteristics of dentine and enamel matrix obtained following processing with the Tooth Transformer® device (S.R.L, Milan, Italy) have been described. It appears that particle size plays a significant role in enhancing soft tissue healing and the body’s resorptive capacity, thereby promoting bone regeneration. In this context, the various devices available on the market enable a consistent particle size between 400–800 μm (Tooth Transformer®), 300–1200 μm (Smart Dentin Grinder®), and 425–1500 μm (Bone Maker®). When none of these devices are utilised, the particle sizes are highly heterogeneous, thereby delaying appropriate tissue healing and regeneration. Although, once the dentine is partially demineralised and the dentinal tubules are widened, osteoclasts can more readily release the organic content from within, inducing the differentiation of osteoblasts. With a particle size between 800–1000 μm, better bone formation results are achieved than with sizes of 426–600 μm, while results are very poor with particles of 180–212 μm35-38.
Apart from its potential to reduce costs, various cultural and ethnic factors may come into direct conflict with different types of biomaterials, such as xenografts and allografts, which, according to recent studies, appear to have the highest rates of rejection by papatients, conferring another important advantage to the tooth when utilised39.
In the clinical case presented, the autologous tooth graft demonstrated good clinical, radiographic and histomorphometric behaviour following a lateral approach maxillary sinus lift with delayed placement of two implants, despite the follow-up period being only six months after prosthodontic restoration; therefore, studies evaluating other important parameters, such as marginal bone loss, over the long term are required.
The use of autologous tooth as a biomaterial in lateral maxillary sinus lift represents an alternative to other biomaterials, exhibiting excellent biocompatibility, a low rate of intraoperative complications, and good patient acceptance. It demonstrates a favourable radiographic appearance over time, although the follow-up period in this clinical case is only six months after prosthetic loading of the implants. The behaviour of the implants in the regenerated bone using tooth as a biomaterial exhibits good clinical and radiographic outcomes, despite the short period since prosthetic loading.
Chappuis V, Araújo MG, Buser D. Clinical relevance of dimensional bone and soft tissue alterations post-extraction in esthetic sites. Periodontol 2000 2017;
73 (1):73–83.
Fischer KR, Gotz W, Kauffmann F, Schmidlin PR, Friedmann A. Ridge preservation of compromised extraction sockets applying a soft cortical membrane: a canine proof-of-principle evaluation. Ann Anat 2020; 231:151524.
Lawson W, Patel ZM, Lin FY. The development and pathologic processes that influence maxillary sinus pneumatization. Anat Rec (Hoboken) 2008; 291(11):1554-1563.
Lana JP, Carneiro PM, Machado Vde C, de Souza PE, Manzi FR, Horta MC. Anatomic variations and lesions of the maxillary sinus detected in cone beam computed tomography for dental implants. Clin Oral Implants Res 2012; 23(12):1398-1403.
Yücesoy T, Göktaş TA. Evaluation of sinus pneumatization and dental implant placement in atrophic maxillary premolar and molar regions. Int J Oral Maxillofac Implants 2022; 37(2):407–415.
Horváth A, Mardas N, Mezzomo LA, Needleman IG, Donos N. Alveolar ridge preservation. A systematic review. Clin Oral Investig 2013; 17(2): 341-363.
Tsai CF, Pan WL, Pan YP et al. Comparison of 4 sinus augmentation techniques for implant placement with residual alveolar bone height ≤3 mm. Medicine (Baltimore) 2020; 99(46):e23180.
Shah D, Chauhan C, Shah R. Survival rate of dental implant placed using various maxillary sinus floor elevation techniques: A systematic review and meta-analysis. J Indian Prosthodont Soc 2022; 22(3):215-224.
Tatum H Jr. Maxillary and sinus implant reconstructions. Dent Clin North Am 1986; 30 (2):207-229.
Boyne PJ, James RA. Grafting of the maxillary sinus floor with autogenous marrow and bone. J Oral Surg 1980; 38 (8):613-616.
Wallace SS, Tarnow DP, Froum SJ et al. Maxillary sinus elevation by lateral window approach: Evolution of technology and technique. J Evid Based Dent Pract 2012; 12:161-171.
Ohayon L, Taschieri S, Friedmann A, Del Fabbro M. Bone graft displacement after maxillary sinus floor augmentation with or without covering barrier membrane: A retrospective computed tomographic image evaluation. Int J Oral Maxillofac Implants 2019; 34:681‐691.
Sakkas A, Wilde F, Heufelder M, Winter K, Schramm A. Autogenous bone grafts in oral implantology is it still a “gold standard”?. A consecutive review of 279 patients with 456 clinical procedures. Int J Implant Dent 2017; 3:1-23.
Jeong KI, Kim SG, Kim YK, Oh JS, Jeong MA, Park JJ. Clinical study of graft materials using autogenous teeth in maxillary sinus augmentation. Implant Dent 2011; 20:471-475.
Elgali I, Omar O, Dahlin C, Thomsen P. Guided bone regeneration: materials and biological mechanisms revisited. Eur J Oral Sci 2017; 125 (5):315-337.
Alkaabi SA, Alsabri GA, NatsirKalla DS et al. A systematic review on regenerative alveolar graft materials in clinical trials: Risk of bias and meta-analysis. J Plast Reconstr Aesthet Surg. 2022; 75(1):356-365.
Kim YK, Kim SG, Byeon JH et al. Development of a novel bone grafting material using autogenous teeth. Oral Surg Oral Med Oral Pathol Oral Radiol Endod 2010; 109 (4):496-503.
Sánchez-Labrador L, Bazal-Bonelli S, Pérez-González F, Sáez-Alcaide LM, Cortés-Bretón Brinkmann J, Martínez- González JM. Autogenous particulated dentin for alveolar ridge preservation. A systematic review. Ann Anat 2023; 14 (246):152024.
Min BM. Oral biochemistry. Springer Nature, 2023.
Kim YK, Lee J, Um IW et al. Tooth- derived bone graft material. J Korean Assoc Oral Maxillofac Surg 2013; 39 (3):103-111.
Kabir A, Murata M, Kusano K et al. Radiological evaluation of human dentin autografts in Bangladesh. J. Hard Tissue Biol 2014; 23:363–370.
Schmidt-Schultz TH, Schultz M. Intact growth factors are conserved in the extracellular matrix of ancient human bone and teeth: A storehouse for the study of human evolution in health and disease. Biol Chem 2005; 386: 767-776.
Misch C. Maxillary autogenous bone grafting. Dent Clip of North Am 2011; 55: 697-713.
Ramírez Fernández MP, Mazón P, Gehrke SA, Calvo-Guirado JL, De Aza PN. Comparison of two xenograft materials used in sinus lift procedures: material characterization and in vivo behavior. Materials (Basel) 2017;10 (6):623.
Campoy TB. Autologous dentin graft behavior in bone regeneration: two histologies at 5 and 10 months. Int J Periodontics Restorative Dent 2021; 41(6):835-842.
Joshi CP, Dani NH, Khedkar SU. Alveolar ridge preservation using autogenous tooth graft versus beta-tricalcium phosphate alloplast: A randomized, controlled, prospective, clinical pilot study. J Indian Soc Periodontol 2016; 20 (4):429-434.
Pesce P, Menini M, Canullo L et al. Radiographic and histomorphometric evaluation of biomaterials used for lateral sinus augmentation: a systematic review on the effect of residual bone height and vertical graft size on new bone formation and graft shrinkage. J Clin Med 2021; 10 (21):4996.
Khijmatgar S, Del Fabbro M, Tumedei M, Testori T, Cenzato N, Tartaglia GM. Residual bone height and new bone formation after maxillary sinus augmentation procedure using biomaterials: a network meta-analysis of clinical trials. Materials (Basel) 2023; 16(4):1376.
Jun SH, Ahn JS, Lee JI, Ahn KJ, Yun PY, Kim YK. A prospective study on the effectiveness of newly developed autogenous tooth bone graft material for sinus bone graft procedure. J Adv Prosthodont. 2014; 6 (6):528-538.
Minetti E, Palermo A, Contessi M et al. Autologous tooth graft for maxillary sinus augmentation: a multicenter clinical study. Int J Growth Factors Stem Cells Dent 2019; 2 (3):45-51.
La Monaca G, Iezzi G, Cristalli MP, Pranno N, Sfasciotti GL, Vozza I. Comparative histological and histomorphometric results of six biomaterials used in two stage maxillary sinus augmentation model after 6 months healing. Biomed Res Int 2018; 2018:9430989.
Kiliç SC, Guengoermues M, Parlak SN. Histologic and histomorphometric assessment of sinus-floor augmentation with beta-tricalcium phosphate alone or in combination with pure-plateletrich plasma or platelet-rich fibrin: A randomized clinical trial. Clin Implant Dent Relat Res 2017;19 (5):959-967.
Pang K, Lee J, Choi S, Kim Y, Kim B, Lee J. Maxillary sinus augmentation with calcium phosphate double-coated anorganic bovine bone: comparative multicenter randomized clinical trial with histological and radiographic evaluation. Implant Dent 2019; 28 (1):39-45.
Avila-Ortiz G, Neiva R, Galindo Moreno P, Rudek I, Benavides E, Wang HL. Analysis of the influence of residual alveolar bone height on sinus augmentation outcomes. Clin Oral Implants Res 2012; 23(9):1082-1088.
Minetti E, Berardini M, Trisi P. A new tooth processing apparatous allowing to obtain dentin grafts for bone augmentation: The tooth transformer. Open Dent J 2019; 13: 6–14.
Pohl S, Binderman I, Tomac J. Maintenance of alveolar ridge dimensions utilizing an extracted tooth dentin particulate autograft and platelet rich fibrin: a retrospective radiographic cone beam computed tomography study. Materials (Basel) 2020;13(5):1083.
Campoy-Beca T. Fractura vertical: Socket shield e injerto autólogo de dentina. RCOE 2019; 24 (1): 22-30.
Koga T, Minamizato T, Kawai Y et al. Bone regeneration using dentin matrix depends on the degree of demineralization and particle size. PLoS One 2016; 11 (1):e0147235.
Bucchi C, Del Fabbro M, Arias A, Fuentes R, Mendes JM, Ordonneau M. Multicenter study of patients’ preferences and concerns regarding the origin of bone grafts utilized in dentistry. Patient Prefer Adherence 2019; 13:179– 185.

Beca Campoy, Tomás
Private practice in surgery and implants, Madrid. PhD candidate in Surgery and
Odonto-stomatology at the University of Salamanca (USAL).
Sánchez-Labrador, Luis
Honorary Associate Lecturer. Department of Clinical Dental Specialties.
Complutense University of Madrid (UCM).
Cortés-Bretón, Jorge
Associate Lecturer in Oral Surgery. Department of Clinical Dental Specialties. Complutense University of Madrid (UCM).
Blanco Antona, Leticia Alejandra
Associate Lecturer, Department
of Surgery, Faculty of Medicine, University of Salamanca (USAL).
Martínez-González, José María
Senior Lecturer in Maxillofacial Surgery. Faculty of Dentistry. Complutense University of Madrid (UCM).