Update
Published in spanish Científica Dental Vol. 19. Nº 2. 2022 www.cientificadental.es
Assessment of the different therapy options in the clinical management of Burning Mouth Syndrome (BMS)
Introduction: The Burning Mouth Syndrome (BMS), whose definition and pathophysiology continue to be a topic of current debate, which also it does not have a universally accepted guidelines for its therapy. Therefore, the objective of this work is to present an assessment of the different treatments for the clinical management of patients with BMS based on the available scientific evidence, so that its application is evaluated in each specific case.
Material and methods: PubMed (MEDLINE) and The Cochrane Library (Wiley) databases were searched for different treatments of BMS. With the data obtained regarding the effectiveness of each therapeutic modality and the adverse effects it produces, three different therapy lines have been developed.
Results: In the first line of therapy we find chewing gum, LLLT (Low-level laser therapy), lingual protector, psychotherapy, topical clonazepam, ALA (Alpha Lipoic Acid) and Catauma. Conclusion: More research is needed to provide sufficient guidance to clinicians on effective therapeutic modalities and that allow to establish a correct strategy in the BMS management.
The Burning Mouth Syndrome (BMS) has received several definitions since it was first described. The last of these was published in 2020 in the first edition of the International Classification of Orofacial Pain (ICOP) 1 proposed by the International Headache Society (IHS). BMS is defined as a burning sensation or oral dysaesthesia that recurs daily for more than two hours a day in a period greater than three months, with no causal lesions evident on clinical examination or research (Table 1). This condition is classified within the category of “idiopathic orofacial pain”, that is to say, no known cause can be attributed to it. However, in the third edition of the International Classification of Headache Disorders (ICHD- III)2 , proposed by the same society just two years earlier, the BMS is included in “painful cranial nerve injuries and other facial pains”, thus attributing it a neuropathic origin. Miller et al.3 question whether BMS can really be considered as a syndrome, since patients do not always suffer from the consistent set of clinical features (dysgeusia and/ or xerostomia) that would constitute it. They propose as the most appropriate term the Burning Mouth Disorder.
There is currently consensus that the secondary burning to a local or systemic disorder should not be considered BMS. It is not accepted that there is a primary and a secondary BMS. Diagnosis of BMS will not be made until all possible alterations are treated and/or controlled and any other possible etiology of oral burning has been ruled out (Table 2)1,2,4.
The prevalence of BMS is particularly high among middle-aged women, coinciding with the peri- and postmenopause5 period. The symptoms are usually bilateral, although the diagnosis is not ruled out if it is unilateral. The most frequent location of the burning sensation is the anterior two thirds of the tongue, followed by the dorsum and lateral edges, the anterior part of the hard palate, the labial mucosa and the gum1,2.
The pathophysiology of BMS is still unknown, although there is growing evidence suggesting that it could have a neuropathic origin existing alterations in different levels of the central or peripheral nervous system that could be involved in its pathogenesis1,2,6. Three different hypotheses have been proposed about its neuropathic origin: small-fibre peripheral sensory neuropathy; a subclinical neuropathy of the trigeminal system (lingual nerve, mandibular nerve, or complete trigeminal nerve); or a hypofunction of dopaminergic neurons6.
medical disorders with no apparent organic cause (such as fibromyalgia, migraine and temporomandibular disorders) that would be linked by a common central sensitization mechanism, in which there is hypersensitivity to noxious and non-noxious stimuli (hyperalgesia and allodynia). All of these syndromes share multiple symptoms, including pain, fatigue, restless sleep, and psychosocial difficulties8.
The lack of scientific evidence regarding the BMS etiology means that, at present, the therapeutic strategy focuses on burning reduction and an improvement in quality of life, without universally accepted guidelines9.
The different therapeutic options that have been proposed for the management of the symptoms related to BMS can be divided according to their origin, in non-pharmacological or pharmacological, and these according to their application topically or systemically.
The aim of this work is to present to the clinical practice an assessment of the different treatments for the clinical management of patients with BMS based on the scientific evidence available to assess their application in each specific case.
The detailed explanation of the theories about the mechanisms involved in the BMS pathogenesis, as well as the action mechanism of the different treatments which are beyond the objective of this article.
Search strategy and inclusion criteria PubMed (MEDLINE) and The Cochrane Library (Wiley) databases were searched using the combination of MeSH terms and free terms: “Burning Mouth Syndrome” [Mesh] AND (“Therapeutics” [Mesh] OR management OR therapy). The search was completed by manual selection of references cited in related systematic reviews.
We included clinical trials (randomized or not), cohort studies, and case-control studies, with at least 10 participants published in English or Spanish, that evaluated the effectiveness of any therapeutic modality used to treat BMS. There was no restriction on the date of publication. In-vitro or animal studies, case reports and cross-sectional studies were excluded. Of the 609 studies found in the initial search, after applying the inclusion and exclusion criteria and discarding irrelevant articles based on the title and the summary, 56 articles were finally selected.
Data synthesis
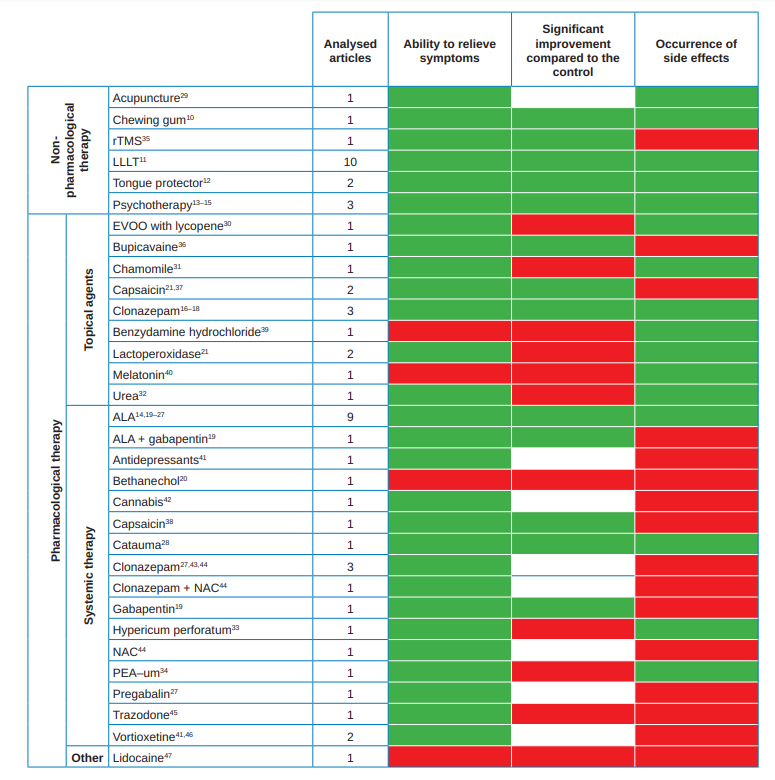
The different therapeutic modalities used for BMS management have been summarized in a table in which the following has been considered:
1. Articles analysed: it is the number of analysed articles for each modality. In the following variables the result reported by the majority has been considered and, in case there were only two articles, the result of the one with a larger sample has been considered.
2. Ability to relieve the symptoms: in case that the therapeutic modality studied has shown an improvement the box is green. The contrary, is shown in red.
3. Significant improvement compared to the control group: favourable data is displayed in green and unfavourable in red. White are those therapeutic modalities that have not been compared with a control group (without data).
4. Appearance of side effects: in green appear those modalities that, if they were compared with a control group, the differences between the two groups were not significant and that, if they were not compared they did not show adverse effects.
In red appear those modalities that, if compared with a control group, showed significantly more adverse effects and, if not compared, showed some adverse effect.
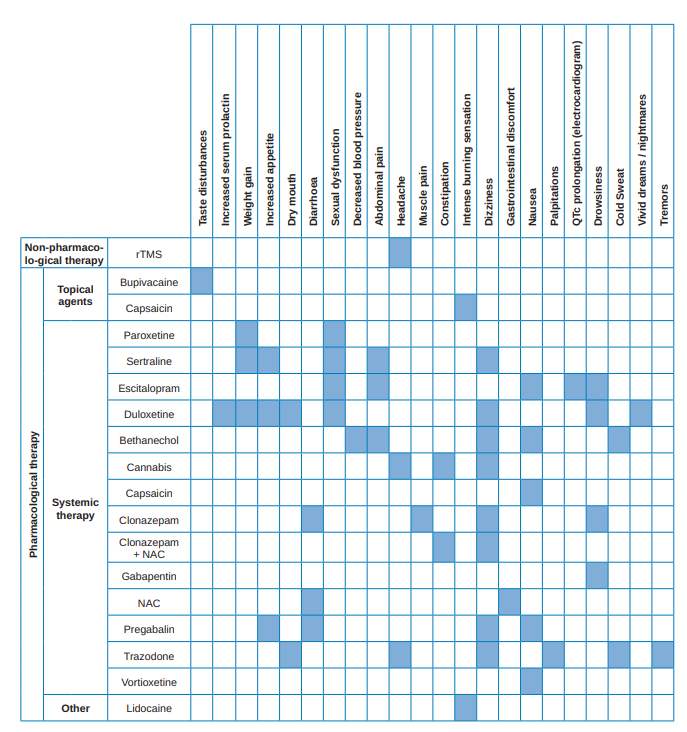
With the results of said table (Table 3), different therapy lines have been proposed. Table 4 shows the side effects of those modalities that had presented them.
Summary tables of therapeutic modalities
With the results obtained in the data evaluation of the available cases, a table has been prepared showing, of each of the therapeutic options, the number of analysed studies and the results of each of them regarding the ability to relieve the symptoms, if this capacity is significant with respect to the control group and the side effects they produce (Table 3). Table 4 summarizes the main secondary effects of those therapeutic modalities that, if compared with a control group, showed significantly more adverse effects and, in case of not being compared showed some adverse effects (i.e., those that have obtained an unfavourable result in the variable “occurrence of side effects” of Table 3).
Lines of therapy
With the data cited above in the tables, several therapy lines have been developed, so that the clinician can assess, in each particular case, the benefit – risk. 1st line of therapy It includes those therapeutic modalities that have shown an improvement in the BMS symptoms, with significant differences regarding the control group and without adverse effects.
Non-pharmacological therapy:
• Chewing gum: chewing unflavoured gum for 20 minutes at a comfortable pace10.
• LLLT (Low-level laser therapy): laser wavelengths, output power, irradiation duration, number of sessions and the radiation frequency varied between 630- 980 nm, 20-300 mW, 10 seconds and 15 minutes, 1 and 20 sessions and 1 to 5 sessions per week, respectively11.
• Tongue protector: single-use transparent plastic, used for 15 minutes 3 times a day for 2 months12.
• Psychotherapy: cognitive therapy (1 or 2 1hr weekly sessions for 2-3 months)13,14 or group psychotherapy (groups of 4 patients, once a week for 3 months)15.
Pharmacological therapy:
• Topical Agents:
– Clonazepam: suck/dissolve tablet of 0.5 or 1mg for 3 minutes in the mouth without swallowing, or rinse with 5ml of solution with 0.1mg/ mL of clonazepam, 3 or 4 times a day16-18.
• Systemic therapy:
– ALA (alpha-lipoic acid; dietary supplement): 200 to 800mg per day for 1 to 2 months14,19-27.
– Catauma (dietary supplement): 2 capsules daily for 2 months. The Catauma contains: Paullinia cupana (125 mg), Trichilia catigua (87.5 mg), Zingiber officinale (10 mg) and Ptychopetalum olacoides (87.5 mg)28. 2nd line of therapy Therapeutic modalities that have shown BMS relief of the symptoms are included, although the differences were not significant with respect to the control group, but without adverse effects. Also, options that despite not having been able to compare them with a control group improved the symptoms and did not show side effects.
Non-pharmacological therapy:
• Acupuncture: Half-hour sessions 3 times a week for 4 weeks at points ST8 (Tou Wei), GB2, TE21, SI19 (Ting Gong), SI18 (Quan Liao), LI4 (Yuan) bilaterally as well
as GV20 (Bai Hui) 29.
Pharmacological therapy:
• Topical Agents:
– EVOO (Extra Virgin Olive Oil) enriched with lycopene: EVOO spray with 300ppm lycopene (SuratTM) 3 times a day30.
– Chamomile: Gel at 2% 2 times a day for 1 month31.
– Lactoperoxidase: mouthwash (BioteneTM) 5 times a day21.
– Urea: at 10% applied topically 3 or 4 times a day for 3 months32.
• Systemic therapy:
– Hypericum perforatum (dietary supplement): 300mg 3 times a day for 3 months33.
– PEA–um (Ultramicronized palmitoylethanolamide; dietary supplement): sublingual dose 600mg 2 times daily for 2 months34. 3rd line of therapy Included are those therapeutic modalities that have been shown to produce BMS symptoms relief, with significant differences from the control group, but which have caused side effects.
Non-pharmacological therapy:
• rTMS (repetitive transcranial magnetic stimulation): 10 sessions of 10Hz pulse series of 5 seconds, at a power intensity of 110% RMT, with an interval between series of 10s during 15 minutes (for a total of 30,000 pulses)35.
Pharmacological therapy:
• Topical Agents:
– Bupivacaine: suck/dissolve tablet 5mg 3 times daily for 2 weeks36.
– Capsaicin: mouthwash at 0.02% 3 times a day for 2 months21,37.
• Systemic therapy:
– ALA + gabapentin (anticonvulsant): 600mg ALA + 300mg gabapentin per day for 2 months19.
– Capsaicin: Capsules at 0.25% 3 times a day38.
– Gabapentin: 300mg per day for 2 months19.
In each particular case, the benefits provided by each therapeutic modality must be weighed with the risks presented and the most appropriate decision made.
Therapy not recommended
These are those therapeutic modalities despite having shown BMS relief of the symptoms, the differences were not significant with respect to the control group, or were not compared with it and also present side effects. Also included are those options that have not shown symptoms relief or have shown conflicting results (such as improvement in one group of patients and worsening in another).
• Pharmacological therapy:
Topical Agents:
– Benzydamine hydrochloride: rinse at 0.15% for 1 minute, 3 times a day for 1 month39.
– Melatonin: compresses applied to oral mucosa with 3mg of melatonin 4 times a day for 2 months40.
• Systemic therapy:
– Antidepressants: Paroxetine (20 mg daily),Sertraline (50 mg daily), Escitalopram (10mg daily), or duloxetine (60 mg daily) for 12 months41.
– Bethanechol (anticholinergic): 15mg per day20.
– Cannabis: 10 to 40 drops of BediolTM (6.3% THC and 8% CBD) 2 times a day42.
– Clonazepam: 0.5 – 2 mg daily for 2 months27,43,44.
– Clonazepam + NAC (N- acetylcysteine, dietary supplement): 0.25mg of clonazepam + 200mg of NAC twice daily for two months44.
– NAC: 200mg twice a day for two months44.
– Pregabalin (anticonvulsant): 150mg a day during 4 months27.
– Trazodone (antidepressant): 200mg 1 time a day for 2 months45.
– Vortioxetine (antidepressant): 10 – 20 mg daily for 12 months41- 46.
Other:
– Lidocaine: lingual nerve block47.
Currently the BMS therapy is mainly symptomatic with the sole aim of relieving the symptoms and improving the quality of life of people who have it. Therefore,
the purpose of this review has been to review the scientific evidence available to develop therapy lines aimed at guiding clinical practice. The different existing therapeutic modalities were evaluated and summarized in Tables 3 and 4.
In the first line of therapy, we have placed all the non-pharmacological therapeutic modalities (except acupuncture, whose study lacked a control group, and rTMS, which showed side effects) and, among the pharmacological modalities, topical clonazepam, ALA and Catauma. All of them have been shown to be effective regarding the ability to relieve symptoms in a meaningful way compared to the control group and without any side effects. Even so, among these treatments, the most studied and, therefore, the most recommended would be LLLT, psychotherapy, ALA and topical clonazepam. Since many professionals do not have laser equipment in their clinics, and that many patients may be reluctant to a psychological therapy for social reasons and the first Cultural15, ALA and topical clonazepam may be the best options to start with. However, it is important to note that before starting to treat a patient, it is essential to make a correct diagnosis and differentiate primary or “real” BMS and secondary burning to another underlying condition.
The diagnostic criteria proposed at the ICOP1 should
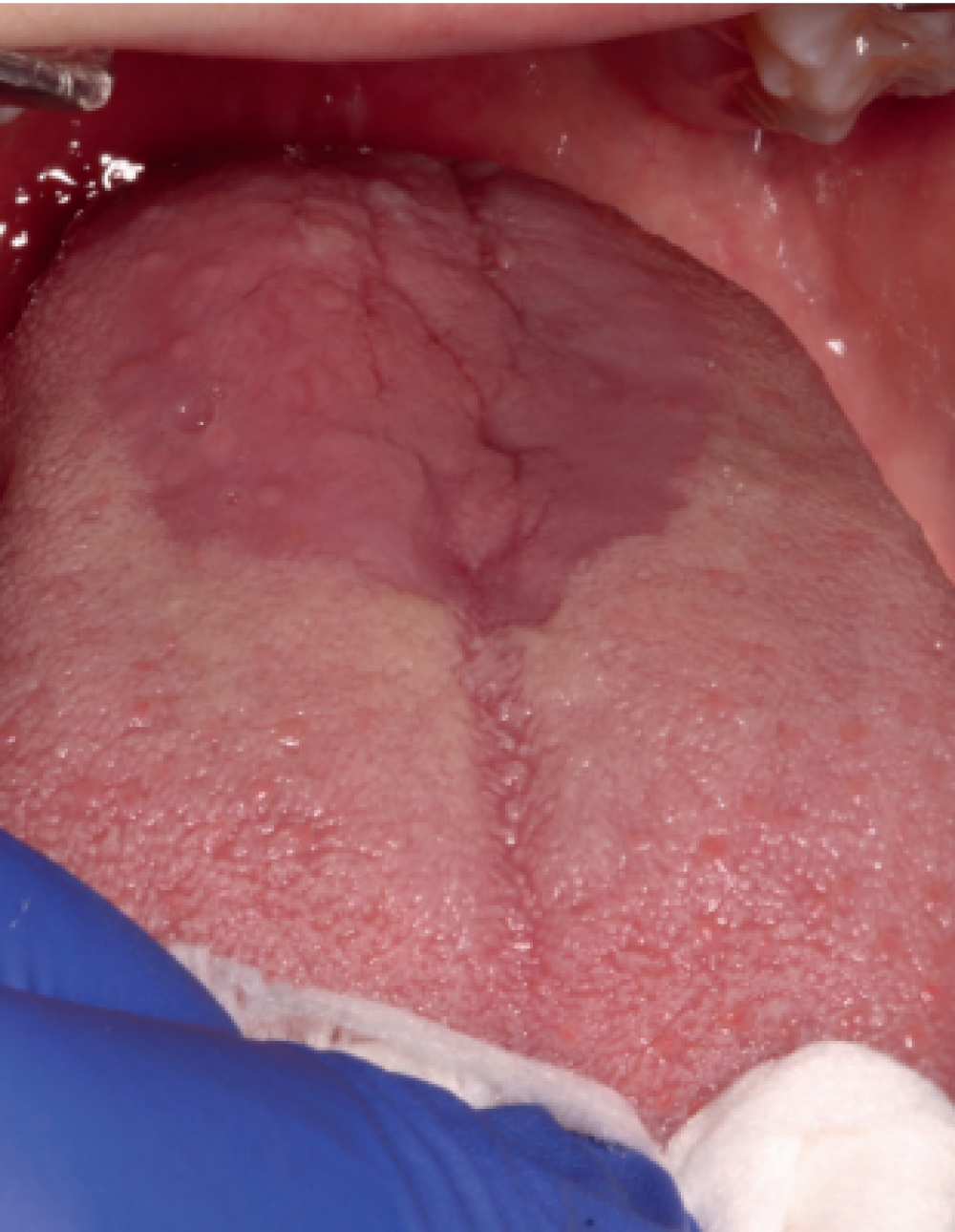
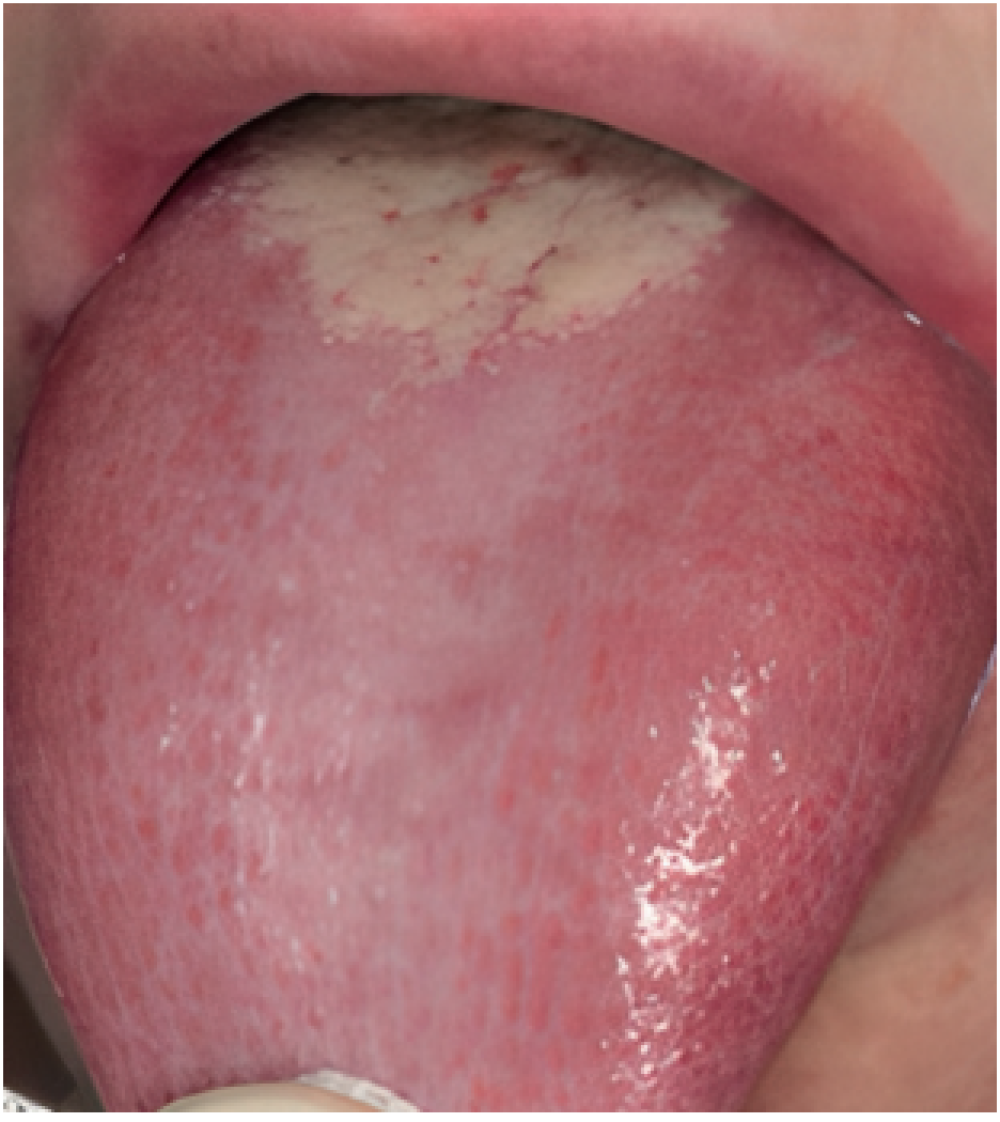
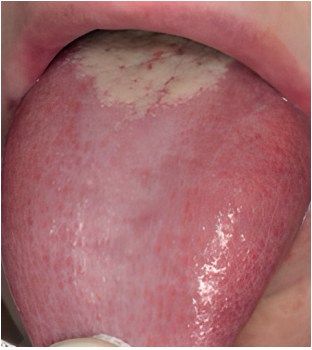
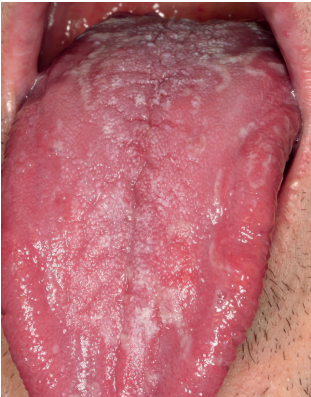
be applied both when selecting cases for new research and before a suspected case in daily clinical practice. One of the criteria of said classification is to exclude other possible local and systemic causes. Therefore, a meticulous differential diagnosis is required. The main pathologies to rule out would be oral lichen planus (Figure 1), geographic tongue (Figure 2) and erythematous candidiasis (Figure 3). All of them have clinical lesions that characterize them and, therefore, can be identified on the examination.
It is advisable to inform patients of the chronic nature of the process and that it lacks the potential for malignancy to reduce anxiety and prevent cancerophobia, which could complicate the condition.
The burning sensation in patients with BMS is exacerbated by the presence of xerostomia31 and many patients report relief when they eat, drink, chew gum or suck some candy. Therefore, even if it is not found as such in the management proposal presented above due to the fact that it is not specific for BMS, intervention will be aimed at improving oral lubrication and hydration, as well as avoiding and/or controlling the possible xerostomia medication. In our opinion, it is possible that some therapeutic modalities that improved the symptoms, but not significantly (second line of therapy), owe their results precisely to a lubricating and moisturizing effect.
The three proposed therapy lines have been developed following the maxim of the medical professions of primum non nocere (above all do no harm). In this sense, therapeutic modalities that may have some side effects have been reserved for those patients who do not respond to the treatments described in the two first therapies. However, considering the possible BMS multifactorial origin (with both central and peripheral causes), certain strategies may be effective in some groups of patients, while they will not be appropriate for others. Therefore, the choice of therapy has to be evaluated individually and should be adapted according to the needs of the patient. In cases where it is considered that the benefits could reduce the risks, as in patients with severe impairment of quality of life, any therapy, both first and third line, should be evaluated.
Some of the side effects mentioned in Table 4 will be more acceptable than others, both due to their medical impact and their intensity. In fact, some authors19,27,35,36,38,42,44,46 mention that their side effects were mild, transient and that in most cases they did not lead to the abandonment of participants in the study.
In the study conducted by Lopez-D’alessandro et al.19, it is mentioned that for the groups treated only with gabapentin and with ALA + gabapentin the secondary effects were present, and although they are described as “very mild”, we consider it an unfavourable outcome for the variable “side effects”, fact that placed both modalities in the third line of therapy. However, the Cochrane48 review mention that, after contacting the authors, only the gabapentin alone group showed significantly more side effects than the control group and, interestingly, the ALA + gabapentin combination did not.
The injection with lidocaine for anaesthesia of the lingual47 nerve is placed in “not recommended treatments” since it presented contradictory results: one group of patients felt a decrease in symptoms and another group felt a worsening or no change in pain (although they felt anaesthetised). When comparing the changes with the VAS (Visual Analogue Scale) before and after the injection in both groups, significant differences were obtained between the two groups.
These curious results, although they cannot be taken as a reference for BMS management in daily practice, they could explore future research on the possible existence of both central and peripheral causes in the BMS pathophysiology.
It is critical to note that the magnitude of the placebo response in BMS appears to be important. Ku-tenShorrer et al49 found that the average placebo response, calculated as a fraction of the active drug response, was 72%. In the daily clinical practice, treatments will not be obfuscated and, therefore, the clinician’s position or opinion of a therapy could modify the patient’s response to the same. It would be important to avoid terms such as “let’s try with…”, “X therapy seems to have…”, etc., since, if the patient perceives that such therapy is not going to be effective, the placebo response could be diminished.
In the scientific literature there are several systematic reviews11,48,50–54, of which the most recent is that of Ślebioda et al.50, in which it was observed that the most effective therapeutic modality was clonazepam (both topical and systemic), and that, in addition, lingual protectors and capsaicin appeared to have promising effects. The Cochrane systematic review by McMillan et al.48 in 2016 concluded that the treatments most supported by scientific evidence for BMS pain relief were, in the short term, photobiomodulation with LLLT, topical clonazepam, lingual protector, and gabapentin.
In the long term, psychotherapy, topical capsaicin, and topical clonazepam would be the most effective. In the present work, only topical capsaicin, gabapentin and systemic clonazepam were placed in the 3rd line of therapy and in treatments not recommended due to the fact that they had significantly more side effects than the control group, or by having presented them without having compared them to the control group.
All others are on the 1st therapy line.
Most of the articles analysed present, in general, a low number of participants, a great heterogeneity in the study design and an important difference in the metrics used to evaluate the results. On the other hand, the existence of different BMS definitions and the lack of standardization in the diagnostic criteria (inclusion/exclusion) may have led to a great variability in case selection. It is important to note that in almost none of the evaluated studies the continuity of the therapeutic effect was analysed once the active therapy was suspended nor the subsequent recurrence of symptoms was analysed. All this has contributed to the lack of rigour of these trials and the disparity of the obtained results, so we currently do not have agreed criteria to manage these patients48,55.
In the scientific literature there are other case reports or pilot studies with other therapeutic modalities, such as Pramipexol56, which have been excluded from this work, but which should be considered for future research increasing the number of cases.
In conclusion, there is currently a need for more studies with a correct choice of cases and an adequate control group, with easily reproducible study designs and longer follow-up periods, and in which the time of the symptoms recurrence after stopping the therapy is evaluated in order to establish an agreed therapeutic algorithm for BMS.
The present narrative review has not analysed the quality or risk of bias of the articles included, nor has it been evaluated whether a therapeutic modality was studied in a single or in several studies, therefore, the results should be considered with caution.
- A correct BMS diagnosis, after the exclusion of other possible conditions that cause similar symptoms will be key to establish an appropriate therapy regimen.
- In the first line of therapy we find chewing gum, LLLT, lingual protector, psychotherapy, topical clonazepam, ALA and Catauma, which are therapeutic modalities that have benefits without side effects, while rTMS, bupivacaine, topical and systemic capsaicin, ALA + gabapentin and gabapentin, located in the third therapy line are also effective, but their side effects should be weighed.
- More research is needed to provide sufficient guidance to clinicians on effective therapeutic modalities that allow to establish a correct strategy in BMS management.
International Classification of Orofacial Pain, 1st edition (ICOP). Cephalalgia 2020;40(2):129–21.
Headache Classification Committee of the International Headache Society (IHS) The International Classification of Headache Disorders, 3rd edition. Cephalalgia 2018;38(1):1–211
Miller CS, Farag AM, Chmieliauskaite
M, y cols. Is burning mouth a syndrome or a disorder? A commentary. Oral Surg Oral Med Oral Pathol Oral Radiol 2019;127(5):361–3.
The IASP classification of chronic pain for ICD-11. Pain. 2018;160(1):88–94.
Kohorst JJ, Bruce AJ, Torgerson RR,
Schenck LA, Davis MD. A populationbased study of the incidence of burning mouth syndrome. Mayo Clin Proc 2014;89(11):1545–52.
Jääskeläinen SK. Pathophysiology of
primary burning mouth syndrome. Clin Neurophysiol 2012;123(1):71–7.
Yunus MB. Editorial Review: An Update on Central Sensitivity Syndromes and the Issues of Nosology and Psychobiology. Curr Rheumatol Rev 2015;11(2):70–85.
Neblett R, Cohen H, Choi Y, Hartell
M, Williams M, Mayer T. The Central
Sensitization Inventory (CSI): establishing clinically significant values for identifying central sensitivity syndromes in an outpatient chronic pain sample. J Pain 2013;14(5):438–45.
Moghadam-Kia S, Fazel N. A diagnostic and therapeutic approach to primary burning mouth syndrome. Clin Dermatol 2017;35(5):453–60.
Sekine N, Okada-Ogawa A, Asano S,
Takanezawa D, Nishihara C, Tanabe N, et al. Analgesic effect of gum chewing in patients with burning mouth syndrome. J Oral Sci 2020;62(4):387–92.
Al-Maweri SA, Javed F, Kalakonda B,
AlAizari NA, Al-Soneidar W, Al-Akwa A. Efficacy of low level laser therapy in the treatment of burning mouth syndrome: A systematic review. Photodiagnosis Photodyn Ther 2017;17:188–93.
López-Jornet P, Camacho-Alonso
F, Andujar-Mateos P. A prospective,
randomized study on the efficacy of tongue protector in patients with burning mouth syndrome. Oral Dis 2011;17(3):277–82.
Bergdahl J, Anneroth G, Ferris H.
Cognitive therapy in the treatment of
patients with resistant burning mouth
syndrome: a controlled study. J Oral
Pathol Med 1995;24(5):213–5.
Femiano F, Gombos F, Scully C.
Burning Mouth Syndrome: Study of
psychotherapy, medication with alphalipoic acid and combination of therapies. Med Oral 2004;9(1):8–13.
Miziara ID, Filho BCA, Oliveira R,
Rodrigues dos Santos RM. Group
psychotherapy: An additional approach to burning mouth syndrome. J Psychosom Res 2009;67(5):443–8.
Gremeau-Richard C, Woda A, Navez ML, y cols. Topical clonazepam in stomatodynia: A randomised placebo-controlled study. Pain 2004;108(1–2):51–7.
Rodríguez de Rivera Campillo E, LópezLópez J, Chimenos-Küstner E. Response to topical clonazepam in patients with burning mouth syndrome: a clinical study.
Bull Group Int Rech Sci Stomatol Odontol 2010;49(1):19–29.
Rossella I, Alessandro V, Naman R, Gary K, Hervé SY. Topical clonazepam for burning mouth syndrome: Is it efficacious in patients with anxiety or depression? J Oral Rehabil 2022;49(1):54–61.
López-D’alessandro E, Escovich L.
Combination of alpha lipoic acid and
gabapentin, its efficacy in the treatment of burning mouth syndrome: A randomized, double-blind, placebo controlled trial. Med
Oral Patol Oral Cir Bucal 2011;16(5).
Femiano F. Burning mouth syndrome
(BMS): an open trial of comparative
efficacy of alpha‐lipoic acid (thioctic acid) with other therapies. Minerva Stomatol 2002;51(9):405–9.
Marino R, Torretta S, Capaccio P,
Pignataro L, Spadari F. Different
therapeutic strategies for burning mouth syndrome: preliminary data. J Oral Pathol Med 2010;39(8):611–6.
Palacios-Sánchez B, Moreno-López LA, Cerero-Lapiedra R, Llamas-Martínez S, Esparza-Gómez G. Alpha lipoic acid efficacy in burning mouth syndrome. A controlled clinical trial. Med Oral Patol Oral Cir Bucal 2015;20(4):e435–40.
Carbone M, Pentenero M, Carrozzo M,
Ippolito A, Gandolfo S. Lack of efficacy
of alpha-lipoic acid in burning mouth syndrome: A double-blind, randomized, placebo-controlled study. Eur J Pain 2009;13(5):492–6
López-Jornet P, Camacho-Alonso F,
Leon-Espinosa S. Efficacy of alpha
lipoic acid in burning mouth syndrome: Arandomized, placebo-treatment study. J Oral Rehabil 2009;36(1):52–7
Cavalcanti DR, Da Silveira FRX. Alpha
lipoic acid in burning mouth syndrome
– A randomized double-blind placebocontrolled trial. J Oral Pathol Med 2009;38(3):254–61
Femiano F, Gombos F, Scully C,
Busciolano M, De Luca P. Burning mouth syndrome (BMS): Controlled open trial of the efficacy of alpha-lipoic acid (thioctic acid) on symptomatology. Oral Dis 2000;6(5):274–7.
Çınar SL, Kartal D, Pergel T, Borlu M.
Effectiveness and safety of clonazepam, pregabalin, and alpha lipoic acid for the treatment of burning mouth syndrome.
Erciyes Med J 2018;40(1):35–8.
Spanemberg JC, Cherubini K, De
Figueiredo MAZ, Gomes APN, Campos
MM, Salum FG. Effect of an herbal
compound for treatment of burning mouth syndrome: Randomized, controlled, double-blind clinical trial. Oral Surg Oral Med Oral Pathol Oral Radiol 2012;113(3):373–7
Jurisic Kvesic A, Zavoreo I, Basic Kes V, y cols. The effectiveness of acupuncture versus clonazepam in patients with burning mouth syndrome. Acupunct Med 2015;33(4):289–92.
Cano-Carrillo P, Pons-Fuster A, LópezJornet P. Efficacy of lycopene-enriched virgin olive oil for treating burning mouth syndrome: A double-blind randomised. J Oral Rehabil 2014;41(4):296–305.
Valenzuela S, Pons-Fuster A, LópezJornet P. Effect of a 2% topical chamomile application for treating burning mouth syndrome: a controlled clinical trial. J Oral Pathol Med 2016;45(7):528–33.
Da Silva L, Siqueira J, Teixeira M, Siqueira S. The role of xerostomia in burning mouth syndrome: a case-control study. Arq Neuropsiquiatr 2014;72(2):91–8.
Sardella A, Lodi G, Demarosi F, Tarozzi M, Canegallo L, Carrassi A. Hypericum perforatum extract in burning mouth syndrome: A randomized placebo controlled study. J Oral Pathol Med 2008;37(7):395–401.
Ottaviani G, Rupel K, Gobbo M,
y cols. Efficacy of ultramicronized
palmitoylethanolamide in burning mouth syndrome-affected patients: a preliminary randomized double-blind controlled trial. Clin Oral Investig 2019;23(6):2743–50.
Umezaki Y, Badran BW, Devries WH,
Moss J, Gonzales T, George MS. The
Efficacy of Daily Prefrontal Repetitive
Transcranial Magnetic Stimulation (rTMS) for Burning Mouth Syndrome (BMS): A Randomized Controlled Single-blind Study. Brain Stimul 2016;9(2):234–42.
Treldal C, Jacobsen CB, Mogensen S, y
cols. Effect of a local anesthetic lozenge in relief of symptoms in burning mouth syndrome. Oral Dis 2016;22(2):123–31.
Silvestre FJ, Silvestre-Rangil J, TamaritSantafé C, Bautista D. Application of a capsaicin rinse in the treatment of burning mouth syndrome. Med Oral Patol Oral Cir Bucal 2012;17(1):2–5.
Petruzzi M, Lauritano D, De Benedittis M, Baldoni M, Serpico R. Systemic capsaicin for burning mouth syndrome: Short-term results of a pilot study. J Oral Pathol Med
2004;33(2):111–4.
Sardella A, Uglietti D, Demarosi F, Lodi G, Bez C, Carrassi A. Benzydamine hydrochloride oral rinses in management of burning mouth syndrome: A clinical trial. Oral Surg Oral Med Oral Pathol Oral
Radiol Endod 1999;88(6):683–6.
Varoni E, Lo Faro A, Lodi G, Carrassi A, Iriti M, Sardella A. Melatonin Treatment in Patients with Burning Mouth Syndrome: A Triple-Blind, Placebo-Controlled, Crossover Randomized Clinical Trial. J Oral Facial Pain Headache 2018;32(2):178–88.
Adamo D, Pecoraro G, Coppola N,
Calabria E, Aria M, Mignogna M.
Vortioxetine versus other antidepressants in the treatment of burning mouth syndrome: An open-label randomized trial. Oral Dis 2021;27(4):1022–41.
Gambino A, Cabras M, Panagiotakos
E, y cols. Evaluating the suitability and
potential efficiency of cannabis sativa
oil for patients with primary burning
mouth syndrome: A prospective, openlabel, single-arm pilot study. Pain Med 2021;22(1):142–51.
Heckmann SM, Kirchner E, Grushka M, Wichmann MG, Hummel T. A double-blind study on clonazepam in patients with burning mouth syndrome. Laryngoscope
2012;122(4):813–6.
Han S, Lim JH, Bang J, Cho JH. Use
of a combination of N-acetylcysteine
and clonazepam to treat burning mouth syndrome. Oral Surg Oral Med Oral Pathol Oral Radiol 2021;132(5):532–8.
Tammiala-Salonen T, Forsseii H.
Trazodone in burning mouth pain: A
placebo-controlled, double-blind study. J Prosthet Dent 1999;82(5):578.
Adamo D, Pecoraro G, Aria M, Favia
G, Mignogna MD. Vortioxetine in the
treatment of mood disorders associated with burning mouth syndrome: Results of an open-label, flexible-dose pilot study. Pain Med (United States) 2020;21(1):185–94.
Grémeau-Richard C, Dubray C, AubletCuvelier B, Ughetto S, Woda A. Effect of lingual nerve block on burning mouth syndrome (stomatodynia): A randomized
crossover trial. Pain 2010;149(1):27–32.
McMillan R, Forssell H, Buchanan JA,
Glenny A, Weldon J, Zakrzewska J.
Interventions for treating burning mouth syndrome. Cochrane Database Syst Rev 2016;11(11):CD002779.
Kuten-Shorrer M, Kelley JM, Sonis ST, Treister NS. Placebo effect in burning mouth syndrome: A systematic review. Oral Dis 2014;20(3):1–6.
Ślebioda Z, Lukaszewska-Kuska M,
Dorocka-Bobkowska B. Evaluation of the efficacy of treatment modalities in burning mouth syndrome—A systematic review. J Oral Rehabil 2020;47(11):1435–47.
Liu YF, Kim Y, Yoo T, Han P, Inman
JC. Burning mouth syndrome: a
systematic review of treatments. Oral Dis 2018;24(3):325–34.
de Souza I, Mármora B, Rados P, Visioli F. Treatment modalities for burning mouth syndrome: a systematic review. Clin Oral
Investig 2018;22(5):1893–905
Kisely S, Forbes M, Sawyer E, Black
E, Lalloo R. A systematic review of
randomized trials for the treatment of
burning mouth syndrome. J Psychosom Res 2016;86:39–46.
Cabras M, Gambino A, Broccoletti R,
De Paola S, Sciascia S, Arduino P.
Effectiveness of Nonpharmacologic
Treatments of Burning Mouth Syndrome: A Systematic Review. J Oral Facial Pain Headache 2021;35(3):175–98.
Ariyawardana A, Chmieliauskaite M,
Farag AM, y cols. World Workshop
on Oral Medicine VII: Burning mouth
syndrome: A systematic review of disease definitions and diagnostic criteria utilized in randomized clinical trials. Oral Dis 2019;25(S1):141–56.
Cárcamo Fonfría A, Gómez-Vicente
L, Pedraza MI, Cuadrado-Pérez ML,
Guerrero Peral AL, Porta-Etessam
J. Burning mouth syndrome: clinical
description, pathophysiological approach, and a new therapeutic option. Neurología 2017;32(4):219–23.

Santmartí Oliver, Margalida
Odontologist at the University of Barcelona (UB). Master in Dental Sciences student in the Complutense University of Madrid (UCM).
Domínguez Gordillo, Adelaide Africa
Associate Professor. Department of Public and Maternal and Child health. Faculty of Medicine.
Complutense University of Madrid (UCM).
Madrigal Martínez-Pereda,
Cristina
Contracted professor doctor.
Co-director of Master Oral Surgery and Implantology.
Department of Dental Clinical Specialties. Odontology Faculty.
Complutense University of Madrid (UCM)
Cerero Lapiedra, Rocío
Full university professor. Dental clinical specialties department.
Dentistry Faculty. Complutense University of Madrid (UCM).